PHYCI_587572: An RxLR Effector Gene and New Biomarker in A Recombinase Polymerase Amplification Assay for Rapid Detection of Phytophthora cinnamomi
Abstract
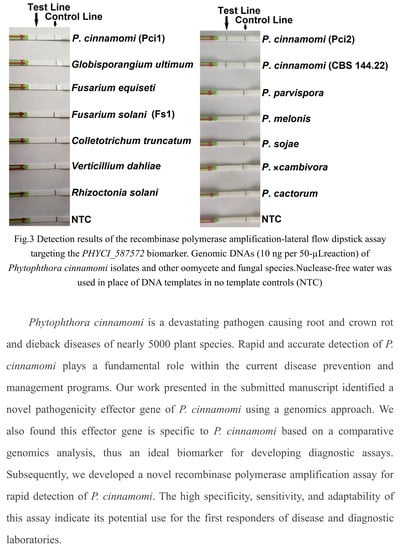
:1. Introduction
2. Materials and Methods
2.1. Isolate Selection and DNA Extraction
2.2. Genomic Sequences
2.3. Identification of A P. cinnamomi-Unique RxLR Effector Gene
2.4. Construction of Binary Potato Virus X (PVX) Vectors
2.5. Agrobacterium Tumefaciens Infiltration
2.6. RPA-LFD Assay
2.7. Evaluation of RPA Specificity and Sensitivity
2.8. Detecting P. cinnamomi in Artificially Inoculated Pine Needles Using RPA-LFD
2.9. Detecting P. cinnamomi in Soil Samples Using the RPA-LFD Assay
3. Results
3.1. Identification of A P. cinnamomi-Unique RxLR Effector Gene PHYCI_587572
3.2. Suppression of Programmed Cell Death by Avh87
3.3. Specificity and Sensitivity of the RPA-LFD Assay
3.4. Detecting P. cinnamomi in Artificial Inoculated Pine Needles
3.5. Detection of P. cinnamomi in Infested Soil Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cahill, D.M.; Rookes, J.E.; Wilson, B.A.; Gibson, L.; McDougall, K.L. Phytophthora cinnamomi and Australia’s biodiversity: Impacts, predictions and progress towards control. Aust. J. Bot. 2008, 56, 279–310. [Google Scholar] [CrossRef]
- Jung, T.; Colquhoun, I.J.; Hardy, G.E.S. New insights into the survival strategy of the invasive soilborne pathogen Phytophthora cinnamomi in different natural ecosystems in Western Australia. For. Pathol. 2013, 43, 266–288. [Google Scholar] [CrossRef]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2018, 19, 260–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, M.D. Phytophthora root rot of avocado. In Plant Diseases of International Importance. Volume III. Diseases of Fruit Crops; Kumar, J., Chaub, H.S., Singh, U.S., Mukhopadhyay, A.N., Eds.; Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Zentmyer, G.A. Phytophthora cinnamomi and the Diseases It Causes; American Phytopathological Society: St. Paul, MN, USA, 1980. [Google Scholar]
- Freinkel, S. American Chestnut: The Life, Death, and Rebirth of a Perfect Tree; University of California Press: Berkeley, CA, USA, 2007. [Google Scholar]
- Shearer, B.L.; Crane, C.E.; Cochrane, A. Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Aust. J. Bot. 2004, 52, 435–443. [Google Scholar] [CrossRef]
- Anderson, P.; Brundrett, M.; Grierson, P.; Robinson, R. Impact of severe forest dieback caused by Phytophthora cinnamomi on macrofungal diversity in the northern jarrah forest of Western Australia. For. Ecol. Manag. 2010, 259, 1033–1040. [Google Scholar] [CrossRef]
- Davis, R.A.; Valentine, L.E.; Craig, M.D.; Wilson, B.; Bancroft, W.J.; Mallie, M. Impact of Phytophthora-dieback on birds in Banksia woodlands in south west Western Australia. Biol. Conserv. 2014, 171, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Lan, C.Z.; Ruan, H.C.; Yao, J.A. First report of Phytophthora cinnamomi causing root and stem rot of blueberry (Vaccinium corymbosum) in China. Plant Dis. 2016, 100, 2537. [Google Scholar] [CrossRef]
- Lan, C.Z.; Ruan, H.C.; Yao, J.A. First report of Phytophthora cinnamomi causing root rot of Castanea mollissima (Chinese chestnut) in China. Plant Dis. 2016, 100, 1248. [Google Scholar] [CrossRef]
- Zheng, X.B.; Lu, J.Y. Studies on Phytophthora species in Fujian, Zhejiang, Jiangsu Provinces and Shanghai, China. Acta Mycol. Sin. 1989, 8, 161–168. [Google Scholar]
- Zeng, H.C.; Ho, H.H.; Zheng, F.C. A survey of Phytophthora species on Hainan Island of South China. J. Phytopathol. 2009, 157, 33–39. [Google Scholar] [CrossRef]
- Bi, X.Q.; Hieno, A.; Otsubo, K.; Kageyama, K.; Liu, G.; Li, M.Z. A multiplex PCR assay for three pathogenic Phytophthora species related to kiwifruit diseases in China. J. Gen. Plant Pathol. 2019, 85, 12–22. [Google Scholar] [CrossRef]
- Zhou, X.G.; Zhu, Z.Y.; Lu, C.P.; Wang, S.J.; Ko, W.H. Phytophthora cinnamomi in Shanghai and it possible origin. Mycopathologia 1992, 120, 29–32. [Google Scholar] [CrossRef]
- Wang, W.J.; Jiao, F.C. Effectors of Phytophthora pathogens are powerful weapons for manipulating host immunity. Planta 2019, 250, 413–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duclos, J.; Fauconnier, A.; Coelho, A.C.; Bollen, A.; Cravador, A.; Godfroid, E. Identification of an elicitin gene cluster in Phytophthora cinnamomi. DNA Seq. 1998, 9, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.L.; Kale, S.D.; Wang, X.; Jiang, R.H.Y.; Bruce, N.A.; Arredondo, F.D.; Zhang, X.M.; Tyler, B.M. RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell 2008, 20, 1930–1947. [Google Scholar] [CrossRef] [Green Version]
- Wawra, S.; Belmonte, R.; Lobach, L.; Saraiva, M.; Willems, A.; van West, P. Secretion, delivery and function of oomycete effector proteins. Curr. Opin. Microbiol. 2012, 15, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.H.Y.; Tripathy, S.; Govers, F.; Tyler, B.M. RXLR effector reservoir in two Phytophthora species is dominated by a single rapidly evolving superfamily with more than 700 members. Proc. Natl. Acad. Sci. USA 2008, 105, 4874–4879. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Hong, C.X.; Richardson, P.A. Rapid detection of Phytophthora cinnamomi using PCR with primers derived from the Lpv putative storage protein genes. Plant Pathol. 2003, 52, 681–693. [Google Scholar] [CrossRef]
- Schena, L.; Duncan, J.M.; Cooke, D.E.L. Development and application of a PCR-based ‘molecular tool box’ for the identification of Phytophthora species damaging forests and natural ecosystems. Plant Pathol. 2008, 57, 64–75. [Google Scholar] [CrossRef]
- Trzewik, A.; Nowak, K.J.; Orlikowska, T. A simple method for extracting DNA from rhododendron plants infected with Phytophthora spp. for use in PCR. J. Plant Prot. Res. 2016, 56, 104–109. [Google Scholar] [CrossRef]
- Williams, N.; Hardy, G.E.S.J.; O’Brien, P.A. Analysis of the distribution of Phytophthora cinnamomi in soil at a disease site in Western Australia using nested PCR. For. Pathol. 2009, 39, 95–109. [Google Scholar] [CrossRef]
- Langrell, S.R.; Morel, O.; Robin, C. Touchdown nested multiplex PCR detection of Phytophthora cinnamomi and P. cambivora from French and English chestnut grove soils. Fungal Biol. 2011, 115, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, J.; Duong, T.A.; van den Berg, N. Development of a nested quantitative real-time PCR for detecting Phytophthora cinnamomi in Persea americana rootstocks. Plant Dis. 2013, 97, 1012–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, T.D.; Martin, F.N.; Coffey, M.D. Development of rapid isothermal amplification assays for detection of Phytophthora spp. in plant tissue. Phytopathology 2015, 105, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Kunadiya, M.B.; Dunstan, W.D.; White, D.; Hardy, G.E.S.J.; Grigg, A.H.; Burgess, T.I. A qPCR assay for the detection of Phytophthora cinnamomi including an mRNA protocol designed to establish propagule viability in environmental samples. Plant Dis. 2019, 103, 2443–2450. [Google Scholar] [CrossRef]
- Dai, T.T.; Yang, X.; Hu, T.; Li, Z.Y.; Xu, Y.; Lu, C.C. A novel LAMP assay for the detection of Phytophthora cinnamomi utilizing a new target gene identified from genome sequences. Plant Dis. 2019, 103, 3101–3107. [Google Scholar] [CrossRef]
- Kunadiya, M.B.; White, D.; Dunstan, W.A.; Hardy, G.E.S.J.; Andjic, V.; Burgess, T.I. Pathways to false-positive diagnoses using molecular genetic detection methods; Phytophthora cinnamomi a case study. FEMS Microbiol. Lett. 2017, 364, fnx009. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Tyler, B.M.; Hong, C.X. An expanded phylogeny for the genus Phytophthora. IMA Fungus 2017, 8, 355–384. [Google Scholar] [CrossRef] [Green Version]
- Scanu, B.; Hunter, G.C.; Linaldeddu, B.T.; Franceschini, A.; Maddau, L.; Jung, T. A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. For. Pathol. 2014, 44, 1–20. [Google Scholar]
- Martin, F.N.; Blair, J.E.; Coffey, M.D. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet. Biol. 2014, 66, 19–32. [Google Scholar] [CrossRef]
- Rojas, J.A.; Miles, T.D.; Coffey, M.D.; Martin, F.N.; Chilvers, M.I. Development and application of qPCR and RPA genus- and species-specific detection of Phytophthora sojae and P. sansomeana root rot pathogens of soybean. Plant Dis. 2017, 101, 1171–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.T.; Yang, X.; Hu, T.; Xu, Y.; Zheng, X.B.; Jiao, B.; Shen, D.Y. Comparative evaluation of a novel recombinase polymerase amplification-lateral flow dipstick (RPA-LFD) assay, LAMP, conventional PCR, and leaf-disc baiting methods for detection of Phytophthora sojae. Front. Microbiol. 2019, 10, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Shen, D.; Dai, T.; Lu, X.; Xu, H.; Dou, D. Rapid and equipment-free detection of Phytophthora capsici using lateral flow strip-based recombinase polymerase amplification assay. Lett. Appl. Microbiol. 2019, 69, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.T.; Hu, T.; Yang, X.; Shen, D.Y.; Jiao, B.B.; Tian, W.; Xu, Y. A recombinase polymerase amplification-lateral flow dipstick assay for rapid detection of the quarantine citrus pathogen in China, Phytophthora hibernalis. PeerJ 2019, 7, e8083. [Google Scholar] [CrossRef] [Green Version]
- James, A.; Macdonald, J. Recombinase polymerase amplification: Emergence as a critical molecular technology for rapid, low-resource diagnostics. Expert Rev. Mol. Diagn. 2015, 15, 1475–1489. [Google Scholar] [CrossRef] [Green Version]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase polymerase amplification for diagnostic applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Nikitin, R.; Haridas, S.; Kuo, A.; Ohm, R.; Otillar, R.; Riley, R.; Salamov, A.; Zhao, X.L.; Korzeniewski, F.; et al. MycoCosm portal: Gearing up for 1000 fungal genomes. Nucl. Acids Res. 2014, 42, D699–D704. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Nielsen, H.; von Heijne, G.; Brunak, S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004, 340, 783–795. [Google Scholar] [CrossRef]
- Haas, B.J.; Kamoun, S.; Zody, M.C.; Jiang, R.H.Y.; Handsaker, R.E.; Cano, L.M.; Grabherr, M.; Kodira, C.D.; Raffaele, S.; Torto-Alalibo, T.; et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 2009, 461, 393–398. [Google Scholar] [CrossRef]
- Whisson, S.C.; Boevink, P.C.; Moleleki, L.; Avrova, A.O.; Morales, J.G.; Gilroy, E.M.; Armstrong, M.R.; Grouffaud, S.; van West, P.; Chapman, S.; et al. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 2007, 450, 115–118. [Google Scholar] [CrossRef]
- Jones, L.; Hamilton, A.J.; Voinnet, O.; Thomas, C.L.; Maule, A.J.; Baulcombe, D.C. RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 1999, 11, 2291–2301. [Google Scholar] [PubMed] [Green Version]
- Hellens, R.P.; Edwards, E.A.; Leyland, N.R.; Bean, S.; Mullineaux, P.M. pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 2000, 42, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.A.; Liu, Z.; Binns, A.N. Three methods for the introduction of foreign DNA into Agrobacterium. In Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 43–54. [Google Scholar]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucl. Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.M.; Qutob, D.; Tedman-Jones, J.; Kuflu, K.; Wang, Y.C.; Tyler, B.M.; Gijzen, M. The Phytophthora sojae avirulence locus Avr3c encodes a multi-copy RXLR effector with sequence polymorphisms among pathogen Strains. PLoS ONE 2009, 4, e5556. [Google Scholar] [CrossRef] [Green Version]





| (Sub) Clade | Species | Isolate | Host or Substrate | Location a | RPA-LFD b |
|---|---|---|---|---|---|
| 7c | Phytophthora cinnamomi | Pci1 | Pinus sp. | AH | + |
| Pci2 | Rhododendron simsii | JS | + | ||
| Pci3 | Cedrus deodara | JS | + | ||
| Pci4 | Camellia oleifera | JS | + | ||
| Pci5 | Pinus sp. | JS | + | ||
| Pci6 | Rhododendron simsii | AH | + | ||
| Pci7 | Rhododendron simsii | SD | + | ||
| Pci8 | Cedrus deodara | SD | + | ||
| Pci9 | Cedrus deodara | AH | + | ||
| Pci10 | Pinus sp. | SD | + | ||
| JP-07-0035 | Pieris sp. | Oregon, USA | + | ||
| PCN-18-001 | Castanopsis sp. | Taiwan | + | ||
| PCN-18-078 | soil | Taiwan | + | ||
| JP-08-313 | Pieris sp. | Oregon, USA | + | ||
| JP-07-0376 | N/A | Oregon, USA | + | ||
| JP-09-325 | N/A | Oregon, USA | + | ||
| ATCC 15400 | Camellia japonica | South Carolina, USA | + | ||
| ATCC 15401 | Persea americana | Puerto Rico | + | ||
| CBS 144.22 | Cinnamomum burmannii | Indonesia | + | ||
| 7c | P. parvispora | CBS132771 | Arbutus unedo | Italy | − |
| CBS132772 | Arbutus unedo | Italy | − | ||
| 7a | P. cambivora | CBS 248.60 | Castanea sativa | USA | − |
| P. fragariae | CBS 209.46 | Fragaria × ananassa | England, UK | − | |
| P. rubi | CBS 967.95 | Rubus idaeus | Scotland, UK | − | |
| 7b | P. melonis | PMNJHG1 | Cucumis sativus | JS | − |
| PMNJHG2 | Cucumis sativus | JS | − | ||
| PMNJHG3 | Cucumis sativus | JS | − | ||
| PMNJDG1 | Benincasa hispida | JS | − | ||
| PMNJDG2 | Benincasa hispida | JS | − | ||
| PMNJDG3 | Benincasa hispida | JS | − | ||
| PMFJHL1 | Lagenaria siceraria | FJ | − | ||
| IMI 325917 | Cucumis sp. | FJ | − | ||
| P. sojae | P6497 | Glycine max | Mississippi, USA | − | |
| Peng-R3 | Glycine max | FJ | − | ||
| 1 | P. cactorum | Pcac1 | Malus pumila | JS | − |
| Pcac2 | Malus pumila | JS | − | ||
| Pcac3 | Rosa chinensis | JS | − | ||
| P. infestans | Pin1 | Solanum tuberosum | FJ | − | |
| Pin2 | Solanum tuberosum | YN | − | ||
| P. nicotianae | Pni1 | Nicotiana tabacum | YN | − | |
| Pni2 | Lycopersicum sp. | JS | − | ||
| Pni3 | Sophora sinensis | JS | − | ||
| Pni4 | Citrus sp. | JS | − | ||
| 2 | P. capsici | Pcap1 | Capsicum annuum | JS | − |
| 3 | P. ilicis | CBS 114348 | Ilex aquifolium | The Netherlands | − |
| 4 | P. palmivora | Ppa1 | Iridaceae | YN | − |
| 4 | P. quercetorum | 15C7 | Soil | South Carolina, USA | − |
| 5 | P. castaneae | CBS 587.85 | Soil | Taiwan | − |
| 6 | P. megasperma | CBS 305.36 | Matthiola incana | California, USA | − |
| P. mississippiae | 57J3 | Irrigation water | Mississippi, USA | − | |
| 8 | P. drechsleri | CBS 292.35 | Beta vulgaris var. altissima | California, USA | − |
| ATCC 56353 | Citrus sinensis | Australia | − | ||
| P. hibernalis | CBS 270.31 | Cirrus sinensis | Setúbal, Portugal | − | |
| P. syringae | ATCC 34002 | Citrus sp. | California, USA | − | |
| P. ramorum | ATCC MYA-2949 | Quercus agrifolia | California, USA | − | |
| 10 | P. boehmeriae | Pbo1 | Boehmeria nivea | JS | − |
| Pbo2 | Gossypium sp. | JS | − | ||
| Pbo3 | Boehmeria nivea | JS | − | ||
| Pbo4 | Gossypium sp. | JS | − | ||
| Oomycete | Globisporangium ultimum | Gu1 | Irrigation water | JS | − |
| Fungi | Alternaria alternata | LH1401 | Cucumis melo | JS | − |
| Aspergillus oryzae | Ao1 | Glycine max | JS | − | |
| Cercospora kikuchii | Ck1 | Glycine max | JS | − | |
| Colletotrichum gloeosporioides | Cg1 | Glycine max | JS | − | |
| C. truncatum | Ct1 | Glycine max | JS | − | |
| Diaporthe phaseolorum var. caulivora | DPC | Glycine max | JS | − | |
| Fusarium oxysporum | Fo1 | Pinus sp. | JS | − | |
| Fusarium solani | Fs1 | Gossypium sp. | JS | − | |
| Fs2 | Glycine max | JS | − | ||
| Fusarium equiseti | Fe1 | Pinus sp. | JS | − | |
| Magnaporthe grisea | Guy11 | Oryza sativa | French Guiana | − | |
| Nigrospora sphaerica | Ns1 | Glycine max | JS | − | |
| Phakopsora pachyrhizi | Pa1 | Glycine max | JS | − | |
| Phomopsis asparagi | Pas1 | Asparagus officinalis | JS | − | |
| Rhizoctonia solani | Rs1 | Gossypium sp. | JS | − | |
| Verticillium dahliae | Vda1 | Gossypium sp. | JS | − |
| Assay | Name | Sequence (5′–3′) |
|---|---|---|
| PVX construction | PHYCI_587572-PVX-HA(infusion)-F | CTAGCATCGATTCCCGGGATGCTCTCGATGACCACAGCCTCC |
| PHYCI_587572-PVX-HA(infusion)-R | CTCTAGAGGATCCCCGGGGAAATTCTCCTCGCGCGTG | |
| Agrobacterium infiltration | LBa | CAATCACAGTGTTGGCTTGC |
| LBb | GACCCTATGGGCTGTGTTG | |
| RPA | PciRL587572F | GCGAGGCCCTCTCGATGACCACAGCCTCCAACCA |
| PciRL587572R | [Biotin]TTGCTGCAGATATGTGCTGCTTGCCTGGACCATC | |
| PciRL587572P | [FAM]GAGGCAGTCGACGATGATGA[THF]TCCTCCGAAGATTCC[C3-spacer] |
| Sample No. | Location a | Vegetation | Year | Detection of Pcin | |||
|---|---|---|---|---|---|---|---|
| DNAs from Soil | DNAs from Leaf Baits | ||||||
| RPA-LFD | PCR | RPA-LFD | PCR | ||||
| 1 | Hefei, AH | Pinus sp. | 2014 | + | + | + | + |
| 2 | Huaibei, AH | Rhododendron simsii | 2014 | + | + | + | + |
| 3 | Liuan, AH | Cedrus deodara | 2016 | + | - | + | + |
| 4 | Suzhou, AH | Camellia oleifera | 2016 | + | - | + | + |
| 5 | Nanjing, JS | Pinus sp. | 2015 | + | + | + | + |
| 6 | Yancheng, JS | Rhododendron simsii | 2015 | + | - | + | + |
| 7 | Xuzhou, JS | Cedrus deodara | 2016 | + | - | + | + |
| 8 | Tianan, SD | Pinus sp. | 2016 | + | + | + | + |
| 9 | Jinan, SD | Rhododendron simsii | 2017 | + | - | + | + |
| 10 | Xiamen, FJ | Pinus sp. | 2016 | + | + | + | + |
| 11 | Zhangzhou, FJ | Rhododendron simsii | 2016 | + | - | + | + |
| 12 | Kunming, YN | Pinus sp. | 2015 | + | + | + | + |
| 13 | Anning, YN | Rhododendron simsii | 2015 | + | - | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.; Wang, A.; Yang, X.; Yu, X.; Tian, W.; Xu, Y.; Hu, T. PHYCI_587572: An RxLR Effector Gene and New Biomarker in A Recombinase Polymerase Amplification Assay for Rapid Detection of Phytophthora cinnamomi. Forests 2020, 11, 306. https://doi.org/10.3390/f11030306
Dai T, Wang A, Yang X, Yu X, Tian W, Xu Y, Hu T. PHYCI_587572: An RxLR Effector Gene and New Biomarker in A Recombinase Polymerase Amplification Assay for Rapid Detection of Phytophthora cinnamomi. Forests. 2020; 11(3):306. https://doi.org/10.3390/f11030306
Chicago/Turabian StyleDai, Tingting, Aohua Wang, Xiao Yang, Xiaowei Yu, Wen Tian, Yue Xu, and Tao Hu. 2020. "PHYCI_587572: An RxLR Effector Gene and New Biomarker in A Recombinase Polymerase Amplification Assay for Rapid Detection of Phytophthora cinnamomi" Forests 11, no. 3: 306. https://doi.org/10.3390/f11030306