Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases
Abstract
:1. Introduction
2. Metabolic Diseases
3. Microbiota, Immunity and Inflammation
3.1. Gut Microbiota, Immune System and Inflammation
3.2. Gut Microbiota and Metabolic Diseases
4. Dietary Flavanols
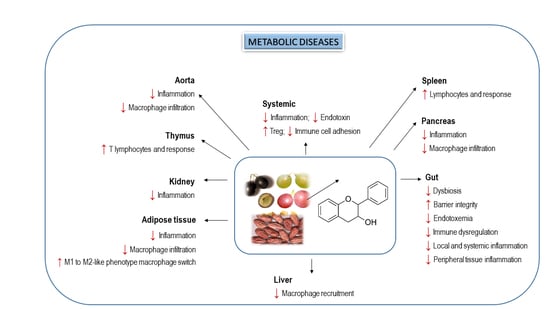
5. Effects of Dietary Flavanols on Immunity and Inflammation in Metabolic Diseases
6. Interplay of Dietary Flavanols and Gut Microbiota in Metabolic Diseases
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hyperten. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, M.C.; Fernandez, M.L. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Derm. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.; Polishchuk, I.; Gorchakova, N.; Zaychenko, H. Metabolic syndrome and lipid metabolism disorders: Molecular and biochemical aspects. Acta Fac. Med. Naissensis 2020, 37, 5–22. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernand, M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr 2016, 7, 66–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J. Diabetes Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of gut microbiota, microbial metabolites and interaction with polyphenol in host immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Ismail, A.; Kersten, S. Molecular mechanisms underlying the potential antiobesity-related diseases effect of cocoa polyphenols. Mol. Nutr. Food Res. 2014, 58, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Andujar, I.; Recio, M.C.; Giner, R.M.; Rıos, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell Longev. 2012. [Google Scholar] [CrossRef]
- Martín, M.A.; Goya, L.; Ramos, S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016, 60, 1756–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, M.A.; Goya, L.; Ramos, S. Protective effects of tea, red wine and cocoa in diabetes. Evidences from human studies. Food Chem. Toxicol. 2017, 109, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Pham, D.V.; Park, P.H. Recent insights on modulation of inflammasomes by adipokines: A critical event for the pathogenesis of obesity and metabolism-associated diseases. Arch. Pharm. Res. 2020, 43, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the metabolic syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Van Treuren, W.; Dodd, D. Microbial contribution to the human metabolome: Implications for health and disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 345–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 2015, 36, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Marrone, M.C.; Coccurello, R. Dietary fatty acids and microbiota-brain. Biomolecules 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 2020, 11, 571731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, P.; Thornberry, N.A.; Pinto, S. The gut-brain axis: Identifying new therapeutic approaches for type 2 diabetes, obesity, and related disorders. Mol. Metab. 2021, 101175. [Google Scholar] [CrossRef]
- Oshima, T.; Miwa, H. Gastrointestinal mucosal barrier function and diseases. J. Gastroenterol. 2016, 51, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and pathogen interaction with the immune system. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Delgado, S.; Sánchez, B.; Margolles, A. Molecular players involved in the interaction between beneficial bacteria and the immune system. Front. Microbiol. 2015, 6, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagg, A.J. Intestinal dendritic cells in health and gut inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postler, T.S.; Ghosh, S. Understanding the holobiont: How microbial metabolites affect human health and shape the immune system. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Ethridge, A.D.; Bazzi, M.H.; Lukacs, N.W.; Huffnagle, G.B. Inter-kingdom communication and regulation of mucosal immunity by the microbiome. J. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kalina, U.; Koyama, N.; Hosoda, T.; Nuernberger, H.; Sato, K.; Hoelzer, D.; Herweck, F.; Manigold, T.; Singer, M.V.; Rossol, S.; et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur. J. Immunol. 2002, 32, 2635–2643. [Google Scholar] [CrossRef]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Joncquel Chevalier-Curt, M.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Lin, M.Y.; De Zoete, M.R.; Van Putten, J.P.; Strijbis, K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front. Immunol. 2015, 6, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernomian, L.; Duarte-Silva, M.; Ribeiro de Barros Cardoso, C. The Aryl Hydrocarbon Receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Proffitt, C.; Bidkhori, G.; Moyes, D.; Shoaie, S. Disease, drugs and dysbiosis: Understanding microbial signatures in metabolic disease and medical interventions. Microorganisms 2020, 8, 1381. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2020. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Delgado, S.; Sánchez, B.; Margolles, A.; Ruas-Madiedo, P.; Ruiz, L. Molecules produced bypProbiotics and intestinal microorganisms with immunomodulatory activity. Nutrients 2020, 12, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ðanić, M.; Stanimirov, B.; Pavlović, N.; Goločorbin-Kon, S.; Al-Salami, H.; Stankov, K.; Mikov, M. Pharmacological applications of bile acids and their derivatives in the treatment of metabolic syndrome. Front. Pharm. 2018, 9, 1382. [Google Scholar] [CrossRef]
- Taleb, S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab. 2018, 28, 737–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. 2020, 21, e12993. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe-generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Zhang, M.; Wang, Z.; Onwuka, J.U.; Wu, X.; Feng, R.; Li, C. Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: A meta-analysis. Acta Diabetol. 2019, 56, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Staahlman, M.; Khan, M.T.; Schmidt, C.; Manneraas-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell 2018, 175, 947–961. [Google Scholar] [CrossRef] [Green Version]
- Molinaro, A.; Lassen, P.B.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.-O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Ramos, S.; Martin, M.A. Impact of diet on gut microbiota. Curr. Opin. Food Sci. 2021, 37, 83–90. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Lamuela-Raventos, R.M.; Santos-Buelga, C.; Sacanella, E.; Castell, M.; Permanyer, J.; Andres-Lacueva, C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009, 394, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef]
- Goya, L.; Martín, M.A.; Sarriá, B.; Ramos, S.; Mateos, R.; Bravo, L. Effect of cocoa and its flavonoids on biomarkers of inflammation: Studies of cell culture, animals and humans. Nutrients 2016, 212. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Massot-Cladera, M.; Franch, A.; Castellote, C.; Castell, M. The effects of cocoa on the immune system. Front. Pharm. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Strat, K.M.; Rowley IV, T.J.; Smithson, A.T.; Tessem, J.S.; Hulver, M.W.; Liu, D.; Davy, B.M.; Davy, K.P.; Neilson, A.P. Mechanisms by which cocoa flavanols improve metabolic syndrome and related disorders. J. Nutr. Biochem. 2016, 35, 1–21. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, P.V.A.; Si, H.; Liu, D. Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-kB-mediated mechanism. Mol. Nutr. Food Res. 2012, 56, 1424–1432. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Chen, X.; Ramos, S.; Devaraj, S. (−)-Epicatechin attenuates high-glucose-induced inflammation by epigenetic modulation in human monocyte. Eur. J. Nutr. 2017, 56, 1369–1373. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary supplementation with a low dose of (−)-epigallocatechin-3-gallate reduces pro-inflammatory responses in peripheral leukocytes of non-obese type 2 diabetic GK rats. J. Nutr. Sci. Vitam. 2013, 59, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, Y.; Suzuki, T.; Mochizuki, K.; Goda, T. Dietary supplementation with (−)-epigallocatechin-3-gallate reduces inflammatory response in adipose tissue of non-obese type 2 diabetic Goto-Kakizaki (GK) rats. J. Agric. Food Chem. 2013, 61, 11410–11417. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E.; Spagnoletta, A.; Magrone, M.; Russo, M.A.; Fontana, S.; Laforgia, F.; Donvito, I.; Campanella, A.; Silvestris, F.; et al. Immune profile of obese people and in vitro effects of red grape polyphenols on peripheral blood mononuclear cells. Oxid. Med. Cell Longev. 2017, 2017, 9210862. [Google Scholar]
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.-C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2010, 34, 800–808. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, T.; Kitadate, K.; Nishioka, H.; Fujii, H.; Kizaki, T.; Kondoh, Y.; Izawa, T.; Ishida, H.; Radák, Z.; Ohno, H. Oligomerized grape seed polyphenols attenuate inflammatory changes due to antioxidative properties in coculture of adipocytes and macrophages. J. Nutr. Biochem. 2010, 21, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-J.; Lo, C.-Y.; Wang, B.-J.; Chiou, R.Y.Y.; Lin, S.M. Theaflavin-3, 3′-digallate, a black tea polyphenol, attenuates adipocyte-activated inflammatory response of macrophage associated with the switch of M1/M2-like phenotype. J. Funct. Foods 2014, 11, 36–48. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Cocoa shell aqueous phenolic extract preserves mitochondrial function and insulin sensitivity by attenuating inflammation between macrophages and adipocytes in vitro. Mol. Nutr. Food Res. 2019, 63, 181413. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-M.; Jialal, I.; Devaraj, S. Effects of epigallocatechin gallate on regulatory T cell number and function in obese v. lean volunteers. Br. J. Nutr. 2010, 103, 1771–1777. [Google Scholar] [CrossRef] [Green Version]
- Zunino, S.J.; Peerson, J.M.; Freytag, T.L.; Breksa 3rd, A.P.; Bonnel, E.L.; Woodhouse, L.R.; Storms, D.H. Dietary grape powder increases IL-1b and IL-6 production by lipopolysaccharide-activated monocytes and reduces plasma concentrations of large LDL and large LDL-cholesterol particles in obese humans. Br. J. Nutr. 2014, 112, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.-H.; Li, M.-Y.; Tsai, M.-L.; Pan, C.-Y.; Badmaev, V.; Ho, C.-T.; La, C.-S. A mixture of citrus polymethoxyflavones, green tea polyphenols and lychee extracts attenuates adipogenesis in 3T3-L1 adipocytes and obesity-induced adipose inflammation in mice. Food Funct. 2019, 10, 7667–7677. [Google Scholar] [CrossRef]
- Peng, J.; Jia, Y.; Hu, T.; Du, J.; Wang, Y.; Cheng, B.; Li, K. GC-(4→8)-GCG, a proanthocyanidin dimer from Camellia ptilophylla, modulates obesity and adipose tissue inflammation in high-fat diet induced obese mice. Mol. Nutr. Food Res. 2019, 63, 1900082. [Google Scholar] [CrossRef]
- Nonino, C.B.; Pinhanelli, V.C.; Noronha, N.Y.; Quinhoneiro, D.C.G.; Souza-Pinhel, M.A.; de Oliveira, B.A.P.; Marchini, J.S.; Ferreira-Nicoletti, C. Green tea supplementation promotes leukocyte telomere length elongation in obese women. Nutr. Hosp. 2018, 35, 570–575. [Google Scholar] [CrossRef]
- Bao, S.; Cao, Y.; Fan, C.; Fan, Y.; Bai, S.; Teng, W.; Shan, Z. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rat. Mol. Nutr. Food Res. 2014, 58, 677–686. [Google Scholar] [CrossRef]
- Gu, Y.; Yu, S.; Park, J.Y.; Harvatine, K.; Lambert, J.D. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. J. Nutr. Biochem. 2014, 25, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.-H.; Kim, H.; Chon, J.-W.; Kim, D.-H.; Nah, S.-Y.; Arvik, T.; Yokoyama, W. Flavonoid-rich Chardonnay grape seed flour supplementation ameliorates diet-induced visceral adiposity, insulin resistance, and glucose intolerance via altered adipose tissue gene expression. J. Funct. Foods 2015, 17, 881–891. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Bao, S.; Yang, W.; Zhang, J.; Li, L.; Shan, Z.; Teng, W. Epigallocatechin gallate prevents inflammation by reducing macrophage infiltration and inhibiting tumor necrosis factor-α signaling in the pancreas of rats on a high-fat diet. Nutr. Res. 2014, 34, 1066–1074. [Google Scholar] [CrossRef]
- Jang, H.-J.; Ridgeway, S.D.; Kim, J. Effects of the green tea polyphenol epigallocatechin-3-gallate on high-fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef]
- Del Bas, J.M.; Crescenti, A.; Arola-Arnal, A.; Oms-Oliu, G.; Arola, L.; Caimari, A. Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int. J. Obes. 2015, 39, 7–15. [Google Scholar] [CrossRef]
- Kataoka, S.; Norikura, T.; Sato, S. Maternal green tea polyphenol intake during lactation attenuates kidney injury in high-fat-diet-fed male offspring programmed by maternal protein restriction in rats. J. Nutr. Biochem. 2018, 56, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Molina, N.; Bolina, A.P.; Otton, R. Green tea polyphenols change the profile of inflammatory cytokine release from lymphocytes of obese and lean rats and protect against oxidative damage. Int. Immunopharmacol. 2015, 28, 985–996. [Google Scholar] [CrossRef]
- Albuquerque, K.F.F.S.; Marinovic, M.P.; Morandi, A.C.; Bolin, A.P.; Otton, R. Green tea polyphenol extract in vivo attenuates inflammatory features of neutrophils from obese rats. Eur. J. Nutr. 2016, 55, 1261–1274. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Mulero, M.; Pinent, M.; Ardévol, A.; Blay, M. Procyanidins and docosahexaenoic acid suppress inflammation and boost immune system in cafeteria diet-fed rats. J. Funct. Foods 2015, 15, 61–71. [Google Scholar] [CrossRef]
- Xu, N.; Chu, J.; Wang, M.; Chen, L.; Zhang, L.; Xie, Z.; Zhang, J.; Ho, C.-T.; Li, D.; Wan, X. Large yellow tea attenuates macrophage-related chronic inflammation and metabolic syndrome in high-fat diet treated mice. J. Agric. Food Chem. 2018, 66, 3823–3832. [Google Scholar] [CrossRef]
- Kumazoe, M.; Nakamura, Y.; Yamashita, M.; Suzuki, T.; Takamatsu, K.; Huang, Y.; Bae, J.; Yamashita, S.; Murata, M.; Yamada, S.; et al. Green tea polyphenol epigallocatechin-3-gallate suppresses Toll-like Receptor 4 expression via up-regulation of E3 ubiquitin-protein ligase RNF216. J. Biol. Chem. 2017, 292, 4077–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhu, X.L.; Sun, Y.K.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X.X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C.; et al. Green tea polyphenols modify the gut microbiome in db/db mice as co-abundance groups correlating with the blood glucose lowering effect. Mol. Nutr. Food Res. 2019, 63, e1801064. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhang, B.; Zheng, W.; Chen, X.; Zhang, J.; Yan, R.; Zhang, T.; Yu, L.; Dong, Y.; Ma, B. Liupao tea extract alleviates diabetes mellitus and modulates gut microbiota in rats induced by streptozotocin and high-fat, high-sugar diet. Biomed. Pharm. 2019, 118, 109262. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; He, X.; Chen, H.; He, Q.; Yao, Z.; Li, Y.; Yang, H.; Simpson, S.J. Oil tea improves glucose and lipid levels and alters gut microbiota in type 2 diabetic mice. Nutr. Res. 2018, 57, 67–77. [Google Scholar] [CrossRef]
- Zhang, H.H.; Liu, J.; Lv, Y.J.; Jiang, Y.L.; Pan, J.X.; Zhu, Y.J.; Huang, M.G.; Zhang, S.K. Changes in intestinal microbiota of type 2 diabetes in mice in response to dietary supplementation with instant tea or matcha. Can. J. Diabetes 2020, 44, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Cilleros, D.; Ramos, S.; López-Oliva, M.E.; Escrivá, F.; Álvarez, C.; Fernández-Millán, E.; Martín, M.A. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020, 132, 109058. [Google Scholar] [CrossRef]
- Tveter, K.M.; Villa-Rodriguez, J.A.; Cabales, A.J.; Zhang, L.; Bawagan, F.G.; Duran, R.M.; Roopchand, D.E. Polyphenol-induced improvements in glucose metabolism are associated with bile acid signaling to intestinal farnesoid X receptor. BMJ Open Diabetes Res. Care 2020, 8, e001386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Guo, H.; He, D.; Zhao, H.; Wang, Z.; Zhang, W.; Liao, L.; Zhang, C.; Ni, L. The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice. Food Funct. 2016, 7, 4869–4879. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef]
- Van Hul, M.; Geurts, L.; Plovier, H.; Druart, C.; Everard, A.; Ståhlman, M.; Rhimi, M.; Chira, K.; Teissedre, P.L.; Delzenne, N.M.; et al. Reduced obesity, diabetes, and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E334–E352. [Google Scholar] [CrossRef]
- Liu, J.H.; Hao, W.J.; He, Z.Y.; Kwek, E.; Zhao, Y.M.; Zhu, H.Y.; Liang, N.; Ma, K.Y.; Lei, L.; He, W.S.; et al. Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 2019, 10, 2847–2860. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, H.; Zhang, K.; Hossen, I.; Wang, J.; Wang, C.; Xu, D.; Xiao, J.; Cao, Y. Protective effects of grape seed procyanidin extract on intestinal barrier dysfunction induced by a long-term high-fat diet. J. Funct. Foods 2020, 64, 103663. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Dey, P.; Sasaki, G.Y.; Wei, P.; Li, J.; Wang, L.; Zhu, J.; McTigue, D.; Yu, Z.; Bruno, R.S. Green tea extract prevents obesity in male mice by alleviating gut dysbiosis in association with improved intestinal barrier function that limits endotoxin translocation and adipose inflammation. J. Nutr. Biochem. 2019, 67, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened Pu-erh tea extract protects mice from obesity by modulating gut microbiota composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, e2000149. [Google Scholar] [CrossRef]
- Sheng, L.; Jena, P.K.; Liu, H.X.; Hu, Y.; Nagar, N.; Bronner, D.N.; Settles, M.L.; Bäumler, A.J.; Wan, Y.Y. Obesity treatment by epigallocatechin-3-gallate-regulated bile acid signaling and its enriched Akkermansia muciniphila. FASEB J. 2018, 32, fj201800370R. [Google Scholar] [CrossRef] [Green Version]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Xing, Y.W.; Lei, G.T.; Wu, Q.H.; Jiang, Y.; Huang, M.X. Procyanidin B2 protects against diet-induced obesity and non-alcoholic fatty liver disease via the modulation of the gut microbiota in rabbits. World, J. Gastroenterol. 2019, 25, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo Flores, M.E. Cocoa flavanols: Natural agents with attenuating effects on metabolic syndrome risk factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2019, 17, 100495. [Google Scholar] [CrossRef]



| Reference | Cellular Model | Flavanol/Food | Concentration | Duration | Main Outcomes |
|---|---|---|---|---|---|
| [68] | HAEC cells + 25 mM glucose | EGCG | 1 µM | 72 h | ↓monocyte adhesion to HAEC, ↓NF-κB. |
| [69] | THP-1 cells (human monocytes) + 25 mM glucose | EC | 5 µM | 24 h | ↓TNF-α, ↓NF-κB, ↓acetyl CBP/p300, ↓HDAC4, ↓H3K9ac/H3, ↓H3K4m2/H3, ↑H3K9me2/H3 |
| [72] | PBMC (from 20 obese volunteers) induced with PMA | Red grape polyphenolic extract | 1, 3, 5 µg/mL | 24 h (co-treatment) | ↓IL-21, ↓IL-1β, ↓IL-6, =IL-17, =TNF-α, ↑IL-10, =IFN-γ, =IL-4, =IL-2 |
| [73] | Macrophages LPS-induced (U937 monocytes) | GPE | 0, 30, 60, 100 µg/mL | 4 h (GPE, 1 h + 100 ng/mL LPS, 3 h) | ↓TNF-α, ↓IL-6, ↓IL-1β, ↓IL-8, ↓IP-10, ↓MCP-1, ↓COX-2, ↓JNK, ↓p38, ↓NF-κB, ↓cJun, ↓Elk-1, ↑IκBα |
| [73] | Adipocytes (abdominal WAT from obese volunteers) induced with macrophage-conditioned media | GPE | 0, 30, 60, 100 µg/mL | 4 h (GPE, 1 h + 100 ng/mL LPS, 3 h) | ↓IL-6, ↓IL-8, ↓MCP-1, ↓IL-1β, ↓NF-κB, ↓glucose uptake in adipocytes |
| [74] | Human white differentiated adipocytes+RAW264 macrophages (co-culture) | Oligomerized grape seed polyphenols | 10, 20 µg/mL | 24 h | ↓TNF-α, ↓MCP-1, ↓PAI-1, ↓ERK, ↓NF-κB, ↓ROS, =FFA release |
| [75]. | Mouse 3T3L1 differentiated adipocytes + RAW264.7 macrophages (co-culture) | Theflavin-3,3′-digallate | 0, 25, 50 µM | 48 h | ↓NO; ↓TNF-α, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓iNOS, ↓CCR7, ↓CD86, ↓CD80, ↑IL-10, ↑CD206, ↑CD163, ↑arginase-1 ↑PPAR-γ, ↓p-IKK, ↓p-IκB, ↓p-p65-NF-κB, ↓COX-2, ↓p-STAT3, ↓ROS, ↓TG, ↓NEFA, ↓FAS, ↓glycerol, ↑ adiponectin, ↑AMPK. |
| [76] | RAW264.7 macrophages (1 µg/mL LPS) | CAE | 31–500 µg/mL | 24 h | ↓NO, ↓PGE2, ↓TNF-α, ↓MCP-1, ↓IL-6, ↓ROS. |
| [76] | Mouse 3T3L1 adipocytes (conditioned media) | CAE | 31–500 µg/mL | 24 h | ↓TNF-α, ↓MCP-1, ↓IL-6, ↓TG, ↓lipid content, ↑glycerol release, ↑lipase activity, ↑adiponectin, ↑mitochondrial function and content, ↑PGC-1α, ↑UCP-1, ↑glucose uptake, ↑GLUT-4 translocation, ↑IR, ↑PI3K, ↑AKT. |
| [77] | PBMC (12 lean + 12 obese, sex-matched) | EGCG | 20 µM | 24 h | ↓NF-κB, ↑Fox3p-positive Tregs, ↑IL-10, ↑HDCA2 in Tregs and HDAC activity. |
| [78] | PBMC (obeses receiving grape powder for 9 weeks) LPS-stimulated (1 µg/L) | Grape powder | 23 g (2×/day, 3.79 mg PP/day) | 24, 48 and 72 h | ↑IL-1β, ↑IL-6, =IL-8. |
| [79] | Mouse 3T3L1 differentiated adipocytes + RAW264.7 macrophages (co-culture) | GTE+ citrus PMFs+ lychee polyphenols | 10–100 µg/mL | 24 h | ↓IL-6, ↓IL-1β, ↓iNOS, ↑p21, ↑p53, ↑AMPK, ↑cyclinE1, ↓CDK2, ↓proliferation, ↓differentiation, ↓C/EBPs, ↓PPARγ. |
| [80] | Mouse 3T3L1 differentiated adipocytes (5 ng/mL TNF-α, co-treatment) | GC-(4→8)-GCG | 10, 20 µg/mL | 24 h | ↓MCP-1, ↓IL-6, ↓COX-2, ↓TG, ↓lipid content, ↓epididymal, ↓PPARγ, ↓SREBP-1c, ↓C/EBPα, ↓p-JNK, =ERK, ↓p-p38↓p-STAT3, ↓p-IκB, ↑IκB, ↓p-p65-NF-κB |
| Reference | Experimental Model | Flavanol/Food | Dose | Duration | Main Outcomes |
|---|---|---|---|---|---|
| Diabetes | |||||
| [68] | db/db mice | EGCG | 0.1% (of diet) | 8 weeks | ↓monocyte adhesion to endothelial cells, ↓MCP-1, ↓KC, ↓ICAM-1, ↓VCAM-1, ↓NF-κB, ↓BP, ↓Cho, ↓TG. |
| [70] | GK rats (peripheral leukocytes) | EGCG | 0.1%, 0.2% and 0.5% | 25 weeks | ↓mRNA TNF-α, ↓IFN-γ, ↓IL-1β, ↓IL-6, ↓IL-18, ↓MCP-1, ↓CD116, ↓S100A6, ↓8-OHdG, ↓MDA, =CD18, ↓BW, =GLU, ↓INS, ↓TG, =ALT, =AST. |
| [71] | GK rats (mesenteric adipose tissue) | EGCG | 0.1%, 0.2% and 0.5% | 25 weeks | =HbA1c, =CD-18, ↓IL-1β, ↓TNF-α, ↓IL-6, ↓IL-12, ↓IL-18, ↓MCP-1, ↓resistin, ↓PAI-1. |
| Obesity | |||||
| [78] | RCDB-cross over (24 obese, 20–60 y, 16 ♀ + 8 ♂) | Grape powder | 23 g (2×/day, 3.79 mg PP/day) | 9 weeks | =IL-1β, =IL-6, =IL-8, =TNF-α, =sICAM-1, =sVCAM-1, =CRP, =leptin, =serum amyloid A, =BMI, =BW, =antioxidant status (ORAC, oxLDL), ↓large LDL-Cho, ↓LDL particles, =ALT, =AST, =alkaline phosphatase. |
| [81] | CSIS (8 lean ♀ + 10 obese ♀, 27–48 y) | Green tea extract | 1009.6 mg (450.7 mg EGCG) | 8 weeks | ↑telomere length in leukocytes in lean and obese participants, =BW, =BMI, =GLU, =alkaline phosphatase, =HDL-Cho, =TG, =AST, ↓LDL-Cho, ↓total Cho, ↓ALT, ↓GGT. |
| [83] | HFD fed mice (60% Kcal from fat) | Cocoa | 8% (of diet) | 18 weeks | ↓TNF-α, ↓IL-6, ↓iNOS, ↓Emr-1, ↓NF-κB, ↓arachidonic acid, ↓COX-2, ↓phospholipase A2, ↓plasmatic endotoxin, ↓GLP-2, =BW, =food intake, =fat weight, =GLU, ↓TG, ↓FFA, ↓INS, ↓HOMA-IR. |
| [82] | HFD fed rats (60% Kcal from fat) | EGCG | 3.2 g/Kg (of diet) | 16 weeks | ↓TLR4, ↓TRAF6, ↓p-IκB, ↓p-NF-κB, ↓TNF-α, ↓IL-6, ↓macrophage infiltration, ↓CD68, ↓BW, ↓epididymal adipose weight, =food intake, =GLU, ↓FFA, ↓INS, ↓HOMA-IR, ↓p-IRS-1, =IRS-1, ↑PI3K (p85), ↑GLUT4. |
| [79] | HFD fed mice (45% Kcal from fat) | GTE+ citrus PMFs+ lychee polyphenols | 0.1–0.5% (of diet) | 16 weeks | ↓MCP-1, ↓IL-6, ↓macrophage infiltration (↓F4/80, ↓CD11b), ↑CD163, ↑IL-10. |
| [80] | HFD fed mice (60% Kcal from fat) | GC-(4→8)-GCG | 40 and 80 mg/Kg | 8 weeks | ↓MCP-1, ↓IL-6, ↓TNF-α, ↓F4/80, ↓CD11b, ↓BW, =food intake, ↓GLU, ↑glucose tolerance, ↑insulin sensitivity, ↓TG, ↓liver weight, ↓hepatic lipid content, ↓epididymal, inguinal and perirenal fat, ↓adipocyte size, ↑adiponectin, ↓leptin, ↓PPARγ, ↓SREBP-1c, ↓C/EBPα, ↓p-STAT3, ↓p-IκB, ↑IκB, ↓p-p65-NF-κB |
| [84] | HFD fed mice (47% Kcal from fat) | Defatted Chardonnay grape seed flour | 10% (of diet) | 5 weeks | ↓Tnf, ↓Tril, ↓Il7r, ↓Adam 8, ↓Il1rn, ↓H2-M2, ↓Lbp, ↓iNOS, ↓Otop1, ↓TLR4, ↓Igsf6, ↓Cnr2, ↓Msr1, ↓Ncf4, ↓Mmp19, ↓CD68, ↑PPARγ, ↓Cebpb, ↓BW, ↓liver weight, ↓GLU, ↓epididymal adipose tissue weight, ↓leptin. |
| [85] | HFD fed rats (60% Kcal from fat) | Grape-seed procyanidin extract | 1–2 mg/animal | 30 days | ↓TNF-α, ↓CRP, ↓IL-6 (serum and adipose tissue), ↓BW, ↓adiponectin, ↓Emr1, ↓macrophage infiltration, ↓NF-κB (liver), ↓TNF-α (liver). |
| [86] | HFD fed rats (60% Kcal from fat) | EGCG | 3.2 g/Kg (of diet) | 16 weeks | ↓TNF-α, ↓IL-6, ↓macrophage infiltration, ↓CD68, =TLR4, =TRAF6, ↓BW, =food intake, ↓INS, =GLU, ↓HOMA-IR, ↓FFA |
| [87] | HFD fed mice (60% Kcal from fat) | EGCG | 50 mg/Kg/day | 10 weeks | ↓macrophage infiltration, ↓F4/80, ↓BW, =GLU, ↓leptin, ↓INS, ↓QUICKI, ↓F4/80 (adipocytes), ↑p-eNOS, ↑p-IRS-1, ↑p-AKT. |
| [88] | HFD fed rats (60% Kcal from fat) | Grape seed procyanidin extract | 25 mg/Kg bw | 30 days | In the offspring: ↓MCP-1, ↓Ccl3, ↓Cl11, ↓Ccl12, ↓phospholipase A2, ↑complement factor I, ↑complement components, ↑adiposity index ↑number of cells in epidydimal adipose tissue, =GLU, =INS, =leptin, =adiponectin, =TG, =total Cho, =FFA, ↓glycerol. |
| [89] | HFD fed rats (45% Kcal from fat) | Green tea extract | 0.12–0.24% (of diet) | 45 weeks | In the offspring: ↓TNF-α, ↓COX-2, ↓PAI-1, ↓macrophage infiltration (CD-68), ↓TGF-β, ↓fibrosis, ↓BW, ↓GLU, ↓TG. |
| Metabolic syndrome | |||||
| [90] | Cafeteria diet fed rats | Green tea | 500 mg/Kg bw/day | 12 weeks | Lymphocytes: ↓IL-2, ↓IL-6, ↓IL-1β, ↓TNF-α, ↓TLR4, ↑IL-10, =IFNγ, =T-bet, =GATA-3, =Foxp3, ↑IRF4, ↓cell proliferation, ↓hexokinase, ↓G6PDH, ↓ROS, ↑MnSOD, ↑CuSOD, ↑GPx, ↑GR, ↑Nrf2, =CAT, ↓BW, ↓FFA, =leptin, ↑adiponectin, ↓glucose intolerance, ↑insulin sensitivity. |
| [91] | Cafeteria diet fed rats | Green tea | 500 mg/Kg bw/day | 12 weeks | Neutrophils: ↑migration capacity, =phagocytic capacity, ↓TNF-α, ↓IL-6, =IL-1β, ↓TLR4, ↓CD11b, ↓IKK, =NF-κB, ↓MPO, ↑hydrogen peroxide, ↑hypochlorous acid, ↑superoxide anion, ↓CAT, =GPx, =GR, =GSH, =GSSG, ↑GSH/GGSG, =hexoquinase, ↓Nrf2, =leptin receptor B, ↓glucose intolerance. |
| [92] | Cafeteria diet fed rats | Grape seed procyanidin extract | 25 mg/Kg bw | 13 weeks (3 weeks supplementation) | Adipose tissue: ↓F4/80, ↓TNF-α, ↓IL-6, ↑Foxp3, ↑IL-10, ↓iNOS. =BW. Serum: =MCP-1, ↓complement factor 3, =leptin, =adiponectin. Thymocytes (thymus and spleen): =IL-6, ↑IL-10, ↓F4/80, =TNF-α. |
| [93] | HFD fed mice (45% Kcal from fat) | Large yellow tea | 0.5 and 2.5% (w/w) | 12 weeks | ↓number of adipocytes, ↓TNF-α, ↓MCP-1, ↓IFNγ, ↓IL-6, =IL-1β, =IL-4, =IL-10, ↓macrophage infiltration, ↓BW, ↓liver weight, ↓adipose tissue weight, ↓INS, ↓GLU, ↑glucose tolerance, ↑insulin sensitivity, ↓TC, ↓TG, ↓LDL, ↓HDL, ↑adiponectin. |
| Reference | Experimental Model | Treatment | Dose | Time (Weeks) | Metabolic Outcomes | Immunity, Inflammation and Gut Microbiota Outcomes |
|---|---|---|---|---|---|---|
| [108] | Zucker diabetic rats | Cocoa rich diet | 10% (of diet) | 10 | ↓BW, ↓GLU, ↓INS, ↓HbA1c, ↓HOMA-IR, ↑HOMA-B, ↓LDL | |
| [103] | db/db obese mice | Grape polyphenol diet | 10% (of diet) | 10 | =BW, ↓GLU, ↑glucose tolerance | =(ZO-1, Occludin, Mucin 2 and serum LPS); =(TNFα, Il-6 and iNOS in ileum); ↑CA and TCA (PBAs) and ↓SBAs serum levels. ↑(Akkermansia, Blautia, Clostridium); ↓(Anaeroplasma, Ruminococcus, Butyricicoccus Dehalobacterium,, Streptococcus, Dorea, Lactococcus, Oscillospira) |
| [106] | C57BL/6J mice fed with HFD induced obesity | Grape pomace extract | 8.2 (g/Kg bw) | 8 | =BW, ↓GLU, ↓INS, ↑glucose tolerance, ↓NEFAs, = Cho, = TG | =ZO-1, ↑(Occludin, Reg3γ, Lyz1);↓(Integrin alpha X, LBP, MCP1 and macrophages in adipose tissue); ↑(Allobaculum, Roseburia); ↓(Desulfovibrio, Clostridium sensu stricto, Lactococcus) |
| [107] | C57BL/6J mice fed with HFD induced obesity | Green, oolong and black tea water extracts | 1% (w/v) | 28 | ↓BW, ↓INS, ↑glucose tolerance, ↓Cho, = TG | ↓(LPS and IL-6 in plasma); ↑(Lachnospiracea, Ruminococcacea) ↓ (Rikenellaceae, Desulfovibrionaceae) |
| [108] | Wistar rats fed with HFD induced obesity | Grape seed procyanidin extract | 200 (mg/Kg bw) | 13 | ↓BW, = GLU ↓Cho, ↓TG, ↓LDL. ↓HDL | ↑(Occludin, ZO-1); ↓Gut permeability; ↓LPS in serum; ↓(TNF-α, IL-1β and IL-6 in ileum); ↑(CD4+, CD25+ Treg in GALT) ↑(Butyricicoccus, Oscillospira Lachnospiraceae, Ruminococcaceae) ↓(Ruminococcaceae_UCG-005, Bacteroidales S24-7 and Ruminococcus_1) |
| [109] | Canines fed with HFD induced obesity | Green tea polyphenols | 1.92% (g/Kg diet) | 18 | ↓BW | ↓(TNF-α, IL-1β and IL-6 in ileum), ↑TLR4 signaling pathway ↑(Acidaminococcus, Succinivibrio and Citrobacter) ↓(Bacteroides, Fusobacterium and Anaerobiospirillum) |
| [110] | C57BL/6J mice fed with HFD induced obesity | Catechin-rich green tea extract | 2% (of diet) | 8 | ↓BW, ↓GLU, ↓INS, ↓HOMA-IR ↓NEFAs, ↓Cho, ↓TG, | ↑(Occludin, ZO-1); ↓LPS in serum; ↓(TNFα, iNOS, MCP-1), ↓(CD68m TLR4, MyD88) and = CD14 in epidydimal fat; ↓(TNFα and iNOS), ↓(CD14, TLR4) and = MD2 in ileum ↑(Akkermansia, Butyrivibrion, Bifidobacterium); ↓(Lactobacillus, Ruminococcus) |
| [111] | C57BL/6 mice fed with HFD induced obesity | Grape seed procyanidin extract | 300 (mg/Kg bw) | 7 | =BW, ↑glucose tolerance, ↑insulin sensitivity | ↓(TNFα, IL-6 and MCP-1) in plasma; ↓(F4/80, CD68 and MCP-1) in epidydimal fat and liver tissues ↑(Clostridium XIVa, Escherichia/Shigella, Blautia, Flavonifractor, Arthrobacter, Roseburia spp and Roseburia inulinivorans); ↓(Lactococcus and Bacteroides) |
| [112] | C57BL/6N mice fed with HFD induced obesity | Pu-erh tea extract | tea | 0.4% (w/v) | ↓BW, ↓GLU, ↑glucose tolerance, ↓Cho, ↓TG, ↓LDL, ↑HDL | ↑(Occludin, ZO-1);↓LPS in serum; ↓(TNF-α, IL-1β and IL-6 in liver), ↑(Anaerotruncus, Alistipes, Odoribacter, Akkermansia, Blautia, Bacteroides, Parabacteroides and Roseburia); ↓(Bilophila, Leuconostoc, Allobaculum) |
| [113] | C57BL/6Cnc mice fed with HFD induced obesity | Grape extract | 1% (w/v) | 13 | ↓BW, ↓GLU, ↑glucose tolerance, ↑insulin sensitivity, ↓Cho | ↓LPS in serum; ↓(TNF-α, IL-6) in serum; ↓(TNFα, IL-6 and MCP-1) in epidydimal fat and liver tissues. ↑ratio of conjugated/free BA , ↑ratio of secondary/primary BA↑(Bifidobacteria, Akkermansia and Clostridia) ;↓(Bacteroides and Desulfovibrio) |
| [114] | C57BL/6 mice fed with WD | EGCG | 100 (mg/Kg bw) | 8 | ↓BW, = GLU, ↑insulin sensitivity, ↓Cho, ↓TG | ↓LPS in serum; ↓(F4/80, CD36) in adipose and liver; ↓serum BAs, ↑(Enterococcaceae and Verrucomicrobiaceae -mainly A. muciniphila-). ↓(Lachnospiraceae, Desulfovibrionaceae, Bacteroidaceae, Prevotellaceae, Rikenellaceae and Deferribacteraceae) |
| [115] | C57BL/6J mice fed with HFD induced obesity | EGCG | 0.3% (w/w) | 8 | ↓BW, ↓GLU, ↓INS, ↓HOMA-IR, ↓Cho | ↑(Claudin-1, Occludin and ZO-1); ↓LPS in serum; ↓(TNFα, iNOS, MCP-1) and ↓(TLR4, MyD88) in liber; ↓TNFα in intestine. ↑(Ruminococcaceae UBA1819 and Parasutterella); ↓(Ruminiclostridium, Clostridium, Blautia, Roseburia, Acetatifactor, Lachnoclostridium, Lachnospiraceae UCG-006) |
| [115] | C57BL/6J mice fed with HFD induced obesity | Catechin | 0.3% (w/w) | 8 | ↓BW, ↓INS, ↓HOMA-IR, ↓Cho | ↑(Claudin-1, Occludin and ZO-1); ↓LPS in serum; ↓(iNOS, MCP-1) and ↓(TLR4, MyD88) in liber; ↓TNFα in intestine. ↑(Ruminiclostridium 9, Oscillibacter); ↓(Ruminiclostridium, Clostridium, Blautia, Roseburia |
| [116] | New Zealand white rabbits fed with HFD induced obesity | Procyanidin B2 | 150 (mg/Kg bw) | 12 | ↓BW, ↓INS ↓Cho, ↓TG, ↓LDL, ↑HDL | ↓LPS in serum; ↑(Ruminococcus, Bacteroidetes. Akkermansia); ↓(Allobaculum) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, M.Á.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. https://doi.org/10.3390/nu13030850
Martín MÁ, Ramos S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients. 2021; 13(3):850. https://doi.org/10.3390/nu13030850
Chicago/Turabian StyleMartín, María Ángeles, and Sonia Ramos. 2021. "Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases" Nutrients 13, no. 3: 850. https://doi.org/10.3390/nu13030850