Update on Stachybotrys chartarum—Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans
Abstract
:Simple Summary
Abstract
1. Introduction
2. Taxonomic Position
3. Species Description
4. Biological and Ecological Aspects
5. Stachybotrys chartarum as a Biodeterioration Factor
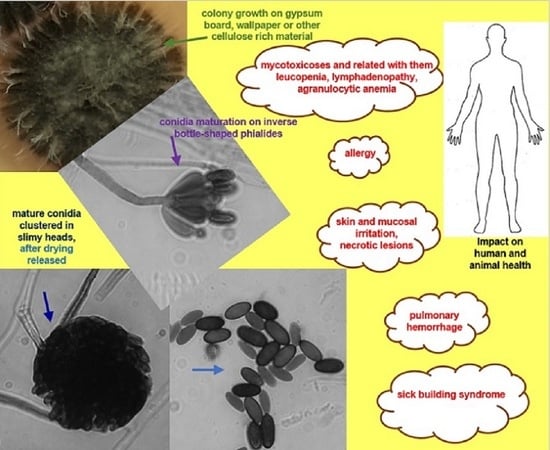
6. Harmful Effects on Humans and Animals Related to the Exposure to Stachybotrys chartarum
7. Antifungal Agents to Eradicate S. chartarum from Indoor Environments
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Jacobs, D.; Mitchell, C.; Miller, D.; Karol, M.H. Improving Indoor Environmental Quality for Public Health: Impediments and Policy Recommendations. Environ. Health Perspect. 2007, 115, 953–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, D.D.; Kalman, D.A. Indoor/Outdoor Air Quality: Reference Pollutant Concentrations in Complaint-Free Residences. Appl. Ind. Hyg. 1989, 4, 17–20. [Google Scholar] [CrossRef]
- Miller, J.D.; McMullin, D.R. Fungal Secondary Metabolites as Harmful Indoor Air Contaminants: 10 Years on. Appl. Microbiol. Biotechnol. 2014, 98, 9953–9966. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Lai, P.S. Indoor Microbial Exposures and Chronic Lung Disease: From Microbial Toxins to the Microbiome. Clin. Chest Med. 2020, 41, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.L.; Horner, W.E.; Kennedy, K.; Grimes, C.; Barnes, C.S.; Phipatanakul, W.; Larenas-Linnemann, D.; Miller, J.D.; Workgroup, E.A. Procedures to Assist Health Care Providers to Determine When Home Assessments for Potential Mold Exposure Are Warranted. J. Allergy Clin. Immunol. Pract. 2016, 4, 417–422.e2. [Google Scholar] [CrossRef] [Green Version]
- Daisey, J.M.; Angell, W.J.; Apte, M.G. Indoor Air Quality, Ventilation and Health Symptoms in Schools: An Analysis of Existing Information. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef]
- Laumbach, R.J.; Kipen, H.M. Bioaerosols and Sick Building Syndrome: Particles, Inflammation, and Allergy. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 135–139. [Google Scholar] [CrossRef]
- Joshi, S.M. The Sick Building Syndrome. Indian J. Occup. Environ. Med. 2008, 12, 61–64. [Google Scholar] [CrossRef]
- Dearborn, D.G.; Yike, I.; Sorenson, W.G.; Miller, M.J.; Etzel, R.A. Overview of Investigations into Pulmonary Hemorrhage among Infants in Cleveland, Ohio. Environ. Health Perspect. 1999, 107 (Suppl. 3), 495–499. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.A. Stachybotrys chartarum (Chartarum = Atra = Alternans) and Other Problems Caused by Allergenic Fungi. Allergy Asthma Proc. 2003, 24, 1–7. [Google Scholar]
- Hossain, M.A.; Ahmed, M.S.; Ghannoum, M.A. Attributes of Stachybotrys chartarum and Its Association with Human Disease. J. Allergy Clin. Immunol. 2004, 113, 200–208, Quiz 209. [Google Scholar] [CrossRef]
- Lombard, L.; Houbraken, J.; Decock, C.; Samson, R.A.; Meijer, M.; Réblová, M.; Groenewald, J.Z.; Crous, P.W. Generic Hyper-Diversity in Stachybotriaceae. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 156–246. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hyde, K.D.; McKenzie, E.H.C.; Jiang, Y.-L.; Li, D.-W.; Zhao, D.-G. Overview of Stachybotrys (Memnoniella) and Current Species Status. Fungal Divers. 2015, 71, 17–83. [Google Scholar] [CrossRef]
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N.V. Comparative Genome Sequencing Reveals Chemotype-Specific Gene Clusters in the Toxigenic Black Mold Stachybotrys. BMC Genom. 2014, 15, 590. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-W.; Yang, C.S. Taxonomic History and Current Status of Stachybotrys chartarum and Related Species. Indoor Air 2005, 15 (Suppl. 9), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.; Hunter, C.A.; Flannigan, B.; Bravery, A.F. The Moisture Requirements of Moulds Isolated from Domestic Dwellings. Int. Biodeterior. 1989, 25, 259–284. [Google Scholar] [CrossRef]
- Piecková, E.; Jesenská, Z. Microscopic Fungi in Dwellings and Their Health Implications in Humans. Ann. Agric. Environ. Med. 1999, 6, 1–11. [Google Scholar] [PubMed]
- Menneer, T.; Mueller, M.; Sharpe, R.A.; Townley, S. Modelling Mould Growth in Domestic Environments Using Relative Humidity and Temperature. Build. Environ. 2022, 208, 108583. [Google Scholar] [CrossRef]
- D’Orazio, M. 12—Materials Prone to Mould Growth. In Woodhead Publishing Series in Civil and Structural Engineering; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 334–350. [Google Scholar] [CrossRef]
- Andersen, B.; Nissen, A.T. Evaluation of Media for Detection of Stachybotrys and Chaetomium Species Associated with Water-Damaged Buildings. Int. Biodeterior. Biodegrad. 2000, 46, 111–116. [Google Scholar] [CrossRef]
- Jagels, A.; Lindemann, V.; Ulrich, S.; Gottschalk, C.; Cramer, B.; Hübner, F.; Gareis, M.; Humpf, H.-U. Exploring Secondary Metabolite Profiles of Stachybotrys spp. by LC-MS/MS. Toxins 2019, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, B.B.; Hinkley, S.F.; Nielsen, K.F. Stachybotrys: An Unusual Mold Associated with Water-Damaged Buildings. Mycotoxin Res. 2000, 16, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; He, Y.; Lin, S.; Zhang, J.; Li, H.; Wang, J.; Hu, Z.; Zhang, Y. Antimicrobial Dolabellanes and Atranones from a Marine-Derived Strain of the Toxigenic Fungus Stachybotrys chartarum. J. Nat. Prod. 2019, 82, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- MMWR Staff. CDC. Update: Pulmonary Hemorrhage/Hemosiderosis among Infants—Cleveland, Ohio. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4909a3.htm (accessed on 7 January 2022).
- Yike, I.; Dearborn, D. Guest Editorial—Novel Insights into the Pathology of Stachybotrys chartarum. Mycopathologia 2011, 172, 1–3. [Google Scholar] [CrossRef]
- Hyde, K.D.; Al-Hatmi, A.M.S.; Andersen, B.; Boekhout, T.; Buzina, W.; Dawson, T.L.; Eastwood, D.C.; Jones, E.B.G.; de Hoog, S.; Kang, Y.; et al. The World’s Ten Most Feared Fungi. Fungal Divers. 2018, 93, 161–194. [Google Scholar] [CrossRef]
- Hughes, S.J. Revisiones Hyphomycetum Aliquot Cum Appen-Dice de Nominibus Rejiciendis. Can. J. Bot. 1958, 36, 727–836. [Google Scholar] [CrossRef]
- Notices, B. Icones Fungorum Hucusque Cognitorum. Auctore A. C. J. Corda. Pragæ, 1837. Ann. Mag. Nat. Hist. 1838, 2, 61–63. [Google Scholar] [CrossRef]
- MYCOBANK Database. Available online: https://www.mycobank.org/ (accessed on 14 February 2022).
- Species Fungorum. Available online: http://www.speciesfungorum.org/Names/SynSpecies.asp?RecordID=306362 (accessed on 14 February 2022).
- Bisby, G.R. Stachybotrys. Trans. Br. Mycol. Soc. 1943, 26, 133–143. [Google Scholar] [CrossRef]
- Koster, B.; Scott, J.; Wong, B.; Malloch, D.; Straus, N. A Geographically Diverse Set of Isolates Indicates Two Phylogenetic Lineages within Stachybotrys chartarum. Can. J. Bot. 2003, 81, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Kong, H. Stachybotrys yunnanensis sp. Nov. and Neosartorya delicata sp. Nov. Isolated from Yunnan, China. Mycotaxon 1997, 62, 427–434. [Google Scholar]
- Andersen, B.; Nielsen, K.F.; Jarvis, B.B. Characterization of Stachybotrys from Water-Damaged Buildings Based on Morphology, Growth, and Metabolite Production. Mycologia 2002, 94, 392–403. [Google Scholar] [CrossRef]
- Andersen, B.; Nielsen, K.F.; Thrane, U.; Szaro, T.; Taylor, J.W.; Jarvis, B.B. Molecular and Phenotypic Descriptions of Stachybotrys chlorohalonata sp. Nov. and Two Chemotypes of Stachybotrys chartarum Found in Water-Damaged Buildings. Mycologia 2003, 95, 1227–1238. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Sutton, D.A.; Li, D.-W.; Liang, Y.; Thompson, E.H.; Wickes, B.L.; Herrera, M.L.; Rhoads, S.L.; Mortensen, J.E. Stachybotrys eucylindrospora Isolated from Foreign Material Following a Traumatic Eye Injury. Mycoses 2014, 57, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Jong, S.C.; Davis, E.E. Contribution to the Knowledge of Stachybotryis and Memnoniella in Culture. Mycotaxon 1976, 3, 409–485. [Google Scholar]
- Haugland, R.A.; Vesper, S.J.; Harmon, S.M. Phylogenetic Relationships of Memnoniella and Stachybotrys Species and Evaluation of Morphological Features for Memnoniella Species Identification. Mycologia 2001, 93, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Vesper, S.; Dearborn, D.G.; Yike, I.; Allan, T.; Sobolewski, J.; Hinkley, S.F.; Jarvis, B.B.; Haugland, R.A. Evaluation of Stachybotrys chartarum in the House of an Infant with Pulmonary Hemorrhage: Quantitative Assessment before, during, and after Remediation. J. Urban Health 2000, 77, 68–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruse, M.; Telerant, R.; Gallagher, T.; Lee, T.; Taylor, J.W. Cryptic Species in Stachybotrys chartarum. Mycologia 2002, 94, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Köck, J.; Gottschalk, C.; Ulrich, S.; Schwaiger, K.; Gareis, M.; Niessen, L. Rapid and Selective Detection of Macrocyclic Trichothecene Producing Stachybotrys chartarum Strains by Loop-Mediated Isothermal Amplification (LAMP). Anal. Bioanal. Chem. 2021, 413, 4801–4813. [Google Scholar] [CrossRef] [PubMed]
- Stielow, J.B.; Lévesque, C.A.; Seifert, K.A.; Meyer, W.; Iriny, L.; Smits, D.; Renfurm, R.; Verkley, G.J.M.; Groenewald, M.; Chaduli, D.; et al. One Fungus, Which Genes? Development and Assessment of Universal Primers for Potential Secondary Fungal DNA Barcodes. Pers. Mol. Phylogeny Evol. Fungi 2015, 35, 242–263. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, A.M.; Peuhkuri, R.H.; Rode, C.; Andersen, B.; Hoof, J.B. Rapid Detection and Identification of Stachybotrys and Chaetomium Species Using Tissue PCR Analysis. J. Microbiol. Methods 2016, 130, 115–122. [Google Scholar] [CrossRef]
- Ulrich, S.; Biermaier, B.; Bader, O.; Wolf, G.; Straubinger, R.K.; Didier, A.; Sperner, B.; Schwaiger, K.; Gareis, M.; Gottschalk, C. Identification of Stachybotrys spp. by MALDI-TOF Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 7565–7581. [Google Scholar] [CrossRef]
- Gruenwald, M.; Rabenstein, A.; Remesch, M.; Kuever, J. MALDI-TOF Mass Spectrometry Fingerprinting: A Diagnostic Tool to Differentiate Dematiaceous Fungi Stachybotrys chartarum and Stachybotrys chlorohalonata. J. Microbiol. Methods 2015, 115, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Niessen, L.; Ekruth, J.; Schäfer, C.; Kaltner, F.; Gottschalk, C. Truncated Satratoxin Gene Clusters in Selected Isolates of the Atranone Chemotype of Stachybotrys chartarum (Ehrenb.) S. Hughes. Mycotoxin Res. 2020, 36, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castlebury, L.A.; Rossman, A.Y.; Sung, G.-H.; Hyten, A.S.; Spatafora, J.W. Multigene Phylogeny Reveals New Lineage for Stachybotrys chartarum, the Indoor Air Fungus. Mycol. Res. 2004, 108, 864–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerbell, R.C.; Gueidan, C.; Schroers, H.-J.; de Hoog, G.S.; Starink, M.; Rosete, Y.A.; Guarro, J.; Scott, J.A. Acremonium Phylogenetic Overview and Revision of Gliomastix, Sarocladium, and Trichothecium. Stud. Mycol. 2011, 68, 139–162. [Google Scholar] [CrossRef]
- Rossman, A.Y.; McKemy, J.M.; Pardo-Schultheiss, R.A.; Schroers, H.-J. Molecular Studies of the Bionectriaceae Using Large Subunit RDNA Sequences. Mycologia 2001, 93, 100–110. [Google Scholar] [CrossRef]
- Xu, J.; Takasaki, A.; Kobayashi, H.; Oda, T.; Yamada, J.; Mangindaan, R.E.P.; Ukai, K.; Nagai, H.; Namikoshi, M. Four New Macrocyclic Trichothecenes from Two Strains of Marine-Derived Fungi of the Genus Myrothecium. J. Antibiot. 2006, 59, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Abbas, H.K.; Johnson, B.B.; Shier, W.T.; Tak, H.; Jarvis, B.B.; Boyette, C.D. Phytotoxicity and Mammalian Cytotoxicity of Macrocyclic Trichothecene Mycotoxins from Myrothecium verrucaria. Phytochemistry 2002, 59, 309–313. [Google Scholar] [CrossRef]
- Sun, T.-T.; Zhu, H.-J.; Cao, F. Chapter 6—The Fungal Myrothecium Genus as a Source of Bioactive Secondary Metabolites. Stud. Nat. Prod. Chem. 2020, 65, 195–237. [Google Scholar] [CrossRef]
- Frazer, S.; Magan, N.; Aldred, D. The Influence of Water Activity and Temperature on Germination, Growth and Sporulation of Stachybotrys chartarum Strains. Mycopathologia 2011, 172, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.J.; Hayden, R.T.; Larone, D.H. Larone’s Medically Important Fungi, 6th ed.; American Society of Microbiology: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- De Silva, L.B.; Herath, W.H.M.W.; Gunawardena, D.S.S.; Wijesundera, R.L.C.; Medis, S.A.; Choudhary, M.I.; Clardy, J. Bisbynin, a Novel Secondary Metabolite from the Fungus Stachybotrys bisbyi (Srinivasan) Barron. Tetrahedron Lett. 1995, 36, 1997–2000. [Google Scholar] [CrossRef]
- Samson, J.R.A.; Houbraken, U.; Thrane, J.C.; Frisvad, B.A. Food and Indoor Fungi; CBS Laboratory Manual Series; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Fassatiová, O. Plísně a Vláknité Houby v Technické Mikrobiologii (Original Title)/Grzyby Mikroskopowe w Mikrobiologii Technicznej (Translation into Polish); Translation, O.H., Ed.; Státní Nakladatelství Tech-Nické Literatury/Wydawnictwa Naukowo-Techniczne: Prague, Czech Republic, 1979. [Google Scholar]
- Ochiai, E.; Kamei, K.; Hiroshima, K.; Watanabe, A.; Hashimoto, Y.; Sato, A.; Ando, A. The Pathogenicity of Stachybotrys chartarum. Nihon Ishinkin Gakkai Zasshi 2005, 46, 109–117. [Google Scholar] [CrossRef]
- Karunasena, E.; Cooley, J.D.; Douglas, D.R.; Straus, D.C. Protein Translation Inhibition by Stachybotrys chartarum Conidia with and without the Mycotoxin Containing Polysaccharide Matrix. Mycopathologia 2004, 158, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B. Stachybotrys chartarum: A Fungus for Our Time. Phytochemistry 2003, 64, 53–60. [Google Scholar] [CrossRef]
- Jarvis, B.B.; Sorenson, W.G.; Hintikka, E.-L.; Nikulin, M.; Zhou, Y.; Jiang, J.; Wang, S.; Hinkley, S.; Etzel, R.A.; Dearborn, D. Study of toxin production by isolates of Stachybotrys chartarum and Memnoniella echinata isolated during a study of pulmonary hemosiderosis in infants. Appl. Environ. Microbiol. 1998, 64, 3620–3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, K.; Stolze, J.L.; Kennedy, A.H.; Money, N.P. Biomechanics of Conidial Dispersal in the Toxic Mold Stachybotrys chartarum. Fungal Genet. Biol. 2007, 44, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Goh, T.K.; Taylor, J.E.; FrÖHlich, J. Byssosphaeria, Chaetosphaeria, Niesslia and Ornatispora Gen. Nov., from Palms. Mycol. Res. 1999, 103, 1423–1439. [Google Scholar] [CrossRef]
- Whitton, S.R.; McKenzie, E.H.C.H.K. Teleomorphic Microfungi Associated with Pandanaceae. In Fungi Associated with Pandanaceae; Springer: Dordecht, The Netherlands; Chiang Rai, Thailand, 2012; pp. 23–124. [Google Scholar] [CrossRef]
- Li, S.; Hartman, G.L.; Jarvis, B.; Tak, H. A Stachybotrys chartarum Isolate from Soybean. Mycopathologia 2002, 154, 41–49. [Google Scholar] [CrossRef]
- Wylke, T.D.; Morehouse, L.G. (Eds.) Mycotoxic Fungi, Mycotoxins, Mycotoxicoses. In Mycotoxic Fungi, Mycotoxins, Mycotoxicoses: An Encyclopedic Handbook. Volume 1: Mycotoxic Fungi and Chemistry of Mycotoxins; Marcel Dekker Inc.: New York, NY, USA, 1977. [Google Scholar]
- Fog Nielsen, K. Mycotoxin Production by Indoor Molds. Fungal Genet. Biol. 2003, 39, 103–117. [Google Scholar] [CrossRef]
- Buerman, E.C.; Worobo, R.W.; Padilla-Zakour, O.I. Thermal Resistance of Xerophilic Fungi in Low-Water-Activity (0.70 to 0.80) Confectionery Model Foods. J. Food Prot. 2019, 82, 390–394. [Google Scholar] [CrossRef]
- Vinnere Pettersson, O.; Leong, S.L. Fungal Xerophiles (Osmophiles); John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Brasel, T.L.; Martin, J.M.; Carriker, C.G.; Wilson, S.C.; Straus, D. Detection of Airborne Stachybotrys chartarum Macrocyclic Trichothecene Mycotoxins in the Indoor Environment. Appl. Environ. Microbiol. 2005, 71, 7376–7388. [Google Scholar] [CrossRef] [Green Version]
- Vašutová, M.; Mleczko, P.; López-García, A.; Maček, I.; Boros, G.; Ševčík, J.; Fujii, S.; Hackenberger, D.; Tuf, I.H.; Hornung, E.; et al. Taxi Drivers: The Role of Animals in Transporting Mycorrhizal Fungi. Mycorrhiza 2019, 29, 413–434. [Google Scholar] [CrossRef]
- Lewis, G.; Harriman, G.; Brundrett, R.K. Humidity Control Design Guide for Commercial and Institutional Buildings; American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE): Atlanta, GA, USA, 2001. [Google Scholar]
- US EPA. Mold Remediation in Schools and Commercial Buildings Guide: Chapter 1. Available online: https://www.epa.gov/mold/mold-remediation-schools-and-commercial-buildings-guide-chapter-1 (accessed on 9 January 2021).
- Pournou, A.; Bogomolova, E. Fungal Colonization on Excavated Prehistoric Wood: Implications for In-Situ Display. Int. Biodeterior. Biodegrad. 2009, 63, 371–378. [Google Scholar] [CrossRef]
- Korpi, A.; Pasanen, A.-L.; Viitanen, H. Volatile Metabolites of Serpula Lacrymans, Coniophora Puteana, Poria Placenta, Stachybotrys chartarum and Chaetomium globosum. Build. Environ. 1998, 34, 205–211. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Hansen, M.Ø.; Larsen, T.O.; Thrane, U. Production of Trichothecene Mycotoxins on Water Damaged Gypsum Boards in Danish Buildings. Int. Biodeterior. Biodegrad. 1998, 42, 1–7. [Google Scholar] [CrossRef]
- Szostak-Kotowa, J. Biodeterioration of Textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Zyska, B. Fungi Isolated from Library Materials: A Review of the Literature. Int. Biodeterior. Biodegrad. 1997, 40, 43–51. [Google Scholar] [CrossRef]
- Drobotko, V.G. Stachybotryotoxicosis. A New Disease of Horses and Humans. Am. Rev. Sov. Med. 1945, 2, 238–242. [Google Scholar]
- Harrach, B.; Bata, A.; Bajmócy, E.; Benko, M. Isolation of Satratoxins from the Bedding Straw of a Sheep Flock with Fatal Stachybotryotoxicosis. Appl. Environ. Microbiol. 1983, 45, 1419–1422. [Google Scholar] [CrossRef] [Green Version]
- Gutarowska, B.; Sulyok, M.; Krska, R. A Study of the Toxicity of Moulds Isolated from Dwellings. Indoor Built Environ. 2010, 19, 668–675. [Google Scholar] [CrossRef]
- Wagner, A.; Hoffman, M.; Green, C.; Barth, E.; Davidson, C.; Gibbs, S.; Scarpino, P. Inactivation of Stachybotrys chartarum Grown on Gypsum Board Using Aerosolized Chemical Agents. J. Environ. Eng. Sci. 2011, 5, 75–79. [Google Scholar] [CrossRef]
- Fantucci, S.; Isaia, F.; Serra, V.; Dutto, M. Insulating Coat to Prevent Mold Growth in Thermal Bridges. Energy Procedia 2017, 134, 414–422. [Google Scholar] [CrossRef]
- Pasanen, A.-L.; Nikulin, M.; Berg, S.; Hintikka, E.-L. Stachybotrys atra Corda May Produce Mycotoxins in Respirator Filters in Humid Environments. Am. Ind. Hyg. Assoc. J. 1994, 55, 62–65. [Google Scholar] [CrossRef]
- Haugland, R.A.; Heckman, J.L. Identification of Putative Sequence Specific PCR Primers for Detection of the Toxigenic Fungal Species Stachybotrys chartarum. Mol. Cell. Probes 1998, 12, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, B. The Fifth Kingdom; Focus Pub.: Newburyport, MA, Canada, 2000. [Google Scholar]
- Jarvis, B.B. Macrocyclic Trichothecenes. In Mycotoxins and Phytoalexins; Sharma, R.P., Salunkhe, D.K.E., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 361–421. [Google Scholar]
- Rashmir-Raven, A.M. Chapter 18—Disorders of the Skin, 4th ed.; Reed, S.M., Bayly, W.M., Sellon, D.C.B.T.-E.I.M., Saunders, W.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1159–1216. [Google Scholar] [CrossRef]
- Nelson, B.D. Stachybotrys chartarum: The Toxic Indoor Mold. Available online: http://www.apsnet.org/online/feature/stachybotrys/ (accessed on 13 January 2021). [CrossRef]
- Hintikka, E.L. Stachybotryotoxicosis as a Veterinary Problem. In Mycotoxins in Human and Animal Health; Rodricks, J.V., Hesseltine, C.W., Mehlman, M.A.E., Eds.; Pathotox Publishers Park Forest South, Ill.: Chicago, IL, USA, 1977; pp. 277–284. [Google Scholar]
- Tantaoui-Elaraki, A.; Mekouar, S.L.; el Hamidi, M.; Senhaji, M. Toxigenic Strains of Stachybotrys atra Associated with Poisonous Straw in Morocco. Vet. Hum. Toxicol. 1994, 36, 93–96. [Google Scholar] [PubMed]
- Mostrom, M.S.; Raisbeck, M.F. Trichothecenes. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 951–976. [Google Scholar]
- Wyllie, T.D.; Morehouse, L.G. Mycotoxicoses of Domestic and Laboratory Animals, Poultry, and Aquatic Invertebrates and Vertebrates; M. Dekker: New York, NY, USA, 1978. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Toxicology of Mycotoxins. EXS 2010, 100, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A.; Bolon, B.; Ochoa, R. Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Miller, J.D.; Rand, T.G.; Jarvis, B.B. Stachybotrys chartarum: Cause of Human Disease or Media Darling? Med. Mycol. 2003, 41, 271–291. [Google Scholar] [CrossRef] [Green Version]
- Sudakin, D.L. Stachybotrys chartarum: Current Knowledge of Its Role in Disease. MedGenMed 2000, 2, E11. [Google Scholar]
- Johanning, E.; Landsbergis, P.; Gareis, M.; Yang, C.S.; Olmsted, E. Clinical Experience and Results of a Sentinel Health Investigation Related to Indoor Fungal Exposure. Environ. Health Perspect. 1999, 107 (Suppl. 3), 489–494. [Google Scholar] [CrossRef] [Green Version]
- Cassimos, C.D.; Chryssanthopoulos, C.; Panagiotidou, C. Epidemiologic Observations in Idiopathic Pulmonary Hemosiderosis. J. Pediatr. 1983, 102, 698–702. [Google Scholar] [CrossRef]
- Elidemir, O.; Colasurdo, G.N.; Rossmann, S.N.; Fan, L.L. Isolation of Stachybotrys from the Lung of a Child with Pulmonary Hemosiderosis. Pediatrics 1999, 104, 964–966. [Google Scholar] [CrossRef]
- Etzel, R.A.; Montaña, E.; Sorenson, W.G.; Kullman, G.J.; Allan, T.M.; Dearborn, D.G.; Olson, D.R.; Jarvis, B.B.; Miller, J.D. Acute Pulmonary Hemorrhage in Infants Associated with Exposure to Stachybotrys atra and Other Fungi. Arch. Pediatr. Adolesc. Med. 1998, 152, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, D.M.; Ghannoum, M.A. Indoor Mold, Toxigenic Fungi, and Stachybotrys chartarum: Infectious Disease Perspective. Clin. Microbiol. Rev. 2003, 16, 144–172. [Google Scholar] [CrossRef] [Green Version]
- Acute Pulmonary Hemorrhage/Hemosiderosis among Infants—Cleveland, January 1993–November 1994. MMWR Morb. Mortal. Wkly. Rep. 1994, 43, 881–883.
- Sorenson, W.G.; Frazer, D.G.; Jarvis, B.B.; Simpson, J.; Robinson, V.A. Trichothecene Mycotoxins in Aerosolized Conidia of Stachybotrys atra. Appl. Environ. Microbiol. 1987, 53, 1370–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, B.B. Chemistry and Toxicology of Molds Isolated from Water-Damaged Buildings BT—Mycotoxins and Food Safety; DeVries, J.W., Trucksess, M.W., Jackson, L.S., Eds.; Springer US: Boston, MA, USA, 2002; pp. 43–52. [Google Scholar] [CrossRef]
- Gareis, M.; Gottschalk, C. Stachybotrys spp. and the Guttation Phenomenon. Mycotoxin Res. 2014, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gregory, L.; Pestka, J.J.; Dearborn, D.G.; Rand, T.G. Localization of Satratoxin-G in Stachybotrys chartarum Spores and Spore-Impacted Mouse Lung Using Immunocytochemistry. Toxicol. Pathol. 2004, 32, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch’s Postulates. Available online: https://mpkb.org/home/pathogenesis/kochs_postulates (accessed on 14 February 2022).
- Croston, T.L.; Lemons, A.R.; Barnes, M.A.; Goldsmith, W.T.; Orandle, M.S.; Nayak, A.P.; Germolec, D.R.; Green, B.J.; Beezhold, D.H. Inhalation of Stachybotrys chartarum Fragments Induces Pulmonary Arterial Remodeling. Am. J. Respir. Cell Mol. Biol. 2019, 62, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Lemons, A.R.; Croston, T.L.; Goldsmith, W.T.; Barnes, M.A.; Jaderson, M.A.; Park, J.-H.; McKinney, W.; Beezhold, D.H.; Green, B.J. Cultivation and Aerosolization of Stachybotrys chartarum for Modeling Pulmonary Inhalation Exposure. Inhal. Toxicol. 2019, 31, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Croft, W.A.; Jarvis, B.B.; Yatawara, C.S. Airborne Outbreak of Trichothecene Toxicosis. Atmos. Environ. 1986, 20, 549–552. [Google Scholar] [CrossRef]
- Vesper, S.J.; Vesper, M.J. Stachylysin May Be a Cause of Hemorrhaging in Humans Exposed to Stachybotrys chartarum. Infect. Immun. 2002, 70, 2065–2069. [Google Scholar] [CrossRef] [Green Version]
- Jagels, A.; Stephan, F.; Ernst, S.; Lindemann, V.; Cramer, B.; Hübner, F.; Humpf, H.-U. Artificial vs Natural Stachybotrys Infestation—Comparison of Mycotoxin Production on Various Building Materials. Indoor Air 2020, 30, 1268–1282. [Google Scholar] [CrossRef]
- Shi, C.; Smith, M.L.; Miller, J.D. Characterization of Human Antigenic Proteins SchS21 and SchS34 from Stachybotrys Chartarum. Int. Arch. Allergy Immunol. 2011, 155, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.; Nielsen, K.F.; Din, S.U. Patterns of Volatile Metabolites and Nonvolatile Trichothecenes Produced by Isolates of Stachybotrys, Fusarium, Trichoderma, Trichothecium and Memnoniella. Environ. Sci. Pollut. Res. 2003, 10, 162. [Google Scholar] [CrossRef]
- Rudert, A.; Portnoy, J. Mold Allergy: Is It Real and What Do We Do about It? Expert Rev. Clin. Immunol. 2017, 13, 823–835. [Google Scholar] [CrossRef]
- Pestka, J.J.; Yike, I.; Dearborn, D.G.; Ward, M.D.W.; Harkema, J.R. Stachybotrys chartarum, Trichothecene Mycotoxins, and Damp Building–Related Illness: New Insights into a Public Health Enigma. Toxicol. Sci. 2008, 104, 4–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordula, T.; Banbula, A.; Macomson, J.; Travis, J. Isolation and Properties of Stachyrase A, a Chymotrypsin-like Serine Proteinase from Stachybotrys chartarum. Infect. Immun. 2002, 70, 419–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, A.; Chidekel, A.S. Acute Pulmonary Hemorrhage in a Delaware Infant after Exposure to Stachybotrys atra. Del. Med. J. 2002, 74, 363–368. [Google Scholar]
- Dearborn, D.G.; Smith, P.G.; Dahms, B.B.; Allan, T.M.; Sorenson, W.G.; Montana, E.; Etzel, R.A. Clinical Profile of 30 Infants with Acute Pulmonary Hemorrhage in Cleveland. Pediatrics 2002, 110, 627–637. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A Global View on Fungal Infections in Humans and Animals: Infections Caused by Dimorphic Fungi and Dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704. [Google Scholar] [CrossRef]
- Ayoubi, N.; Dass, V.L. Black Mold: A Case Presentation and Discussion of Cutaneous Stachybotrys chartarum Infection. Dermatol. Arch. 2019, 3, 80–81. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Semis, M.; Dadwal, S.S.; Tegtmeier, B.R.; Wilczynski, S.P.; Ito, J.I.; Kalkum, M. First Case of Invasive Stachybotrys Sinusitis. Clin. Infect. Dis. 2021, 72, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Seroy, J.; Antiporta, P.; Grim, S.A.; Proia, L.A.; Singh, K.; Clark, N.M. Aspergillus calidoustus Case Series and Review of the Literature. Transpl. Infect. Dis. 2017, 19, e12755. [Google Scholar] [CrossRef] [PubMed]
- Glampedakis, E.; Cassaing, S.; Fekkar, A.; Dannaoui, E.; Bougnoux, M.-E.; Bretagne, S.; Neofytos, D.; Schreiber, P.W.; Hennequin, C.; Morio, F.; et al. Invasive Aspergillosis Due to Aspergillus Section Usti: A Multicenter Retrospective Study. Clin. Infect. Dis. 2021, 72, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Egli, A.; Fuller, J.; Humar, A.; Lien, D.; Weinkauf, J.; Nador, R.; Kapasi, A.; Kumar, D. Emergence of Aspergillus calidoustus Infection in the Era of Posttransplantation Azole Prophylaxis. Transplantation 2012, 94, 403–410. [Google Scholar] [CrossRef]
- Yike, I.; Vesper, S.; Tomashefski, J.F.; Dearborn, D.G. Germination, Viability and Clearance of Stachybotrys chartarum in the Lungs of Infant Rats. Mycopathologia 2003, 156, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Blevins, S.M.; Bronze, M.S. Robert Koch and the ‘Golden Age’ of Bacteriology. Int. J. Infect. Dis. 2010, 14, e744–e751. [Google Scholar] [CrossRef] [Green Version]
- Page, E.H.; Trout, D.B. The Role of Stachybotrys Mycotoxins in Building-Related Illness. AIHAJ Am. Ind. Hyg. Assoc. 2001, 62, 644–648. [Google Scholar] [CrossRef]
- Chung, Y.-J.; Yang, G.-H.; Islam, Z.; Pestka, J.J. Up-Regulation of Macrophage Inflammatory Protein-2 and Complement 3A Receptor by the Trichothecenes Deoxynivalenol and Satratoxin G. Toxicology 2003, 186, 51–65. [Google Scholar] [CrossRef]
- Latgé, J.P. Aspergillus fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, S.; Schäfer, C. Toxin Production by Stachybotrys chartarum Genotype S on Different Culture Media. J. Fungi 2020, 6, 159. [Google Scholar] [CrossRef]
- Aleksic, B.; Bailly, S.; Draghi, M.; Pestka, J.J.; Oswald, I.P.; Robine, E.; Bailly, J.D.; Lacroix, M.Z. Production of Four Macrocyclic Trichothecenes by Stachybotrys chartarum during Its Development on Different Building Materials as Measured by UPLC-MS/MS. Build. Environ. 2016, 106, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Hinkley, S.F.; Mazzola, E.P.; Fettinger, J.C.; Lam, Y.-F.; Jarvis, B.B. Atranones A–G, from the Toxigenic Mold Stachybotrys chartarum. Phytochemistry 2000, 55, 663–673. [Google Scholar] [CrossRef]
- Vesper, S.J.; Magnuson, M.L.; Dearborn, D.G.; Yike, I.; Haugland, R.A. Initial Characterization of the Hemolysin Stachylysin from Stachybotrys chartarum. Infect. Immun. 2001, 69, 912–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.P.; Green, B.J.; Beezhold, D.H. Fungal hemolysins. Med. Mycol. 2013, 51, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Rakkestad, K.E.; Skaar, I.; Ansteinsson, V.E.; Solhaug, A.; Holme, J.A.; Pestka, J.J.; Samuelsen, J.T.; Dahlman, H.J.; Hongslo, J.K.; Becher, R. DNA Damage and DNA Damage Responses in THP-1 Monocytes after Exposure to Spores of Either Stachybotrys chartarum or Aspergillus versicolor or to T-2 Toxin. Toxicol. Sci. 2010, 115, 140–155. [Google Scholar] [CrossRef] [Green Version]
- de Hoog, G.S. Risk Assessment of Fungi Reported from Humans and Animals*. Mycoses 1996, 39, 407–417. [Google Scholar] [CrossRef]
- Zyska, B. Fungi in Indoor Air in European Countries. Mikol. Lek. 2001, 8, 127–140. [Google Scholar]
- Mahmoudi, M.; Gershwin, M. Sick Building Syndrome. III. Stachybotrys chartarum. J. Asthma 2000, 37, 191–198. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Boone, S.; Bright, K.R.; Gerba, C.P. Occurrence of Household Mold and Efficacy of Sodium Hypochlorite Disinfectant. J. Occup. Environ. Hyg. 2012, 9, 663–669. [Google Scholar] [CrossRef]
- Martyny, J.W.; Harbeck, R.J.; Pacheco, K.; Barker, E.A.; Sills, M.; Silveira, L.; Arbuckle, S.; Newman, L. Aerosolized Sodium Hypochlorite Inhibits Viability and Allergenicity of Mold on Building Materials. J. Allergy Clin. Immunol. 2005, 116, 630–635. [Google Scholar] [CrossRef]
- Chakravarty, P.; Kovar, B. Engineering Case Report. J. Occup. Environ. Hyg. 2013, 10, D11–D16. [Google Scholar] [CrossRef] [PubMed]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal Properties of Silver Nanoparticles against Indoor Mould Growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.E.; Hoyano, Y.; Summons, R.E.; Bacon, V.A.; Duffield, A.M. Chlorination Studies II. The Reaction of Aqueous Hypochlorous Acid with α-Amino Acids and Dipeptides. Biochim. Biophys. Acta Gen. Subj. 1973, 313, 170–180. [Google Scholar] [CrossRef]
- Rossoni, E.M.M.; Gaylarde, C.C. Comparison of Sodium Hypochlorite and Peracetic Acid as Sanitising Agents for Stainless Steel Food Processing Surfaces Using Epifluorescence Microscopy. Int. J. Food Microbiol. 2000, 61, 81–85. [Google Scholar] [CrossRef]
- Srinivasan, S.; Velusamy, G.; Munshi, M.A.I.; Radhakrishnan, K.; Tiwari, R.V.C. Comparative Study of Antifungal Efficacy of Various Endodontic Irrigants with and without Clotrimazole in Extracted Teeth Inoculated with Candida Albicans. J. Contemp. Dent. Pract. 2020, 21, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Eggleston, P.A. Allergenic Proteins Are Fragmented in Low Concentrations of Sodium Hypochlorite. Clin. Exp. Allergy 2001, 31, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.S.; Kennedy, K.; Johnson, L.; Forrest, E.; Gard, L.; Pacheco, F.; Amado, M.; Portnoy, J. Use of Dilute Sodium Hypochlorite Spray and Home Cleaning to Reduce Indoor Allergen Levels and Improve Asthma Health Parameters. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2008, 101, 551–552. [Google Scholar] [CrossRef]
- Fu, E.; McCue, K.; Boesenberg, D.F. 1—Chemical Disinfection of Hard Surfaces—Household, Industrial and Institutional Settings; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 573–592. [Google Scholar] [CrossRef]
- Arthur, R. Damp Indoor Spaces and Health; The National Academies Press: Washington, DC, USA, 2004. [Google Scholar] [CrossRef]
- Menetrez, M.Y.; Foarde, K.K.; Webber, T.D.; Dean, T.R.; Betancourt, D.A. Testing Antimicrobial Paint Efficacy on Gypsum Wallboard Contaminated with Stachybotrys chartarum. J. Occup. Environ. Hyg. 2007, 5, 63–66. [Google Scholar] [CrossRef]
- Whiley, H.; Gaskin, S.; Schroder, T.; Ross, K. Antifungal Properties of Essential Oils for Improvement of Indoor Air Quality: A Review. Rev. Environ. Health 2018, 33, 63–76. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [Green Version]
- Šegvić Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal Activity of Thyme (Thymus vulgaris L.) Essential Oil and Thymol against Moulds from Damp Dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Biermaier, B.; Gottschalk, C.; Schwaiger, K.; Gareis, M. Occurrence of Stachybotrys chartarum Chemotype S in Dried Culinary Herbs. Mycotoxin Res. 2015, 31, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Peèiulytë, D. Effect of Tea Tree Essential Oil on Microorganisms 2. Evaluation of Fungal Reaction to Tea Tree Oil under Different Conditions. Biologija 2005, 2, 21–28. [Google Scholar]


| Taxon Name | Current Name | Synonyms |
|---|---|---|
| Kingdom | Fungi | |
| Subkingdom | Dikarya | |
| Phylum | Ascomycota | |
| Subphylum | Pezizomycotina | |
| Class | Sordariomycetes | |
| Subclass | Hypocreomycetidae | |
| Order | Hypocreales | |
| Family | Stachybotryaceae | |
| Genus | Stachybotrys | Fuckelina; Gliobotrys; Hyalobotrys; Hyalostachybotrys; Memnoniella; Spinomyces; Synsporium |
| Currently accepted species 1−4 S. aksuensis; S. albipes; S. aloicola; S. alternans; S. alternans var. alterans; S. alternans var. atoxicus; S. asperulus; S. atra f. genuina; S. atra var. atra; S. atrogriseus; S. atrus; S. atrus f. atrus; S. atrus f. lobatus; S. atrus var. atrus; S. atrus var. brevicaulis; S. atrus var. cylindrosporus; S. atrus var. microsporus; S. aurantius; S. bambusicola; S. biformis; S. bisbyi; S. breviusculus; S. cannae; S. chartarum; S. chlorohalonata; S. clitoriae; S. cordylines; S. crassus; S. cylindrosporus; S. dakotensis; S. dichrous; S. dolichophialis; S. echinatus; S. elasticae; S. elastus; S. elegans; S. elongatus; S. eucylindrospora; S. freycinetiae; S. frondicola; S. gamsii; S. globosus; S. gracilis; S. guttulisporus; S. havanensis; S. humilis; S. indicoides; S. indicus; S. jiangziensis; S. kampalensis; S. kapiti; S. klebahnii; S. leprosus; S. levisporus; S. limonisporus; S. littoralis; S. lobulatus; S. lobulatus var. lobulatus; S. longisporus; S. longistipitatus; S. lunzinensis; S. magniferae; S. mexicanus; S. microsporus; S. mohanramii; S. musae; S. nepalensis; S. nephrodes; S. nephrospora; S. nielamuensis; S. nilagirica; S. oenanthes; S. oleronensis; S. pallescens; S. palmae; S. palmicola; S. palmijunci; S. papyrogena; S. parva; S. parvispora; S. phaeophialis; S. proliferata; S. pulchra; S. punctatus; S. queenslandica; S. ramosa; S. reniformis; S. renispora; S. renisporoides; S. reniverrucosa; S. ruwenzoriensis; S. saccharii; S. sansevieriae; S. sansevieriicola; S. scabra; S. setosa; S. sinuatophora; S. socia; S. sphaerospora; S. stilboidea; S. subcylindrosporus; S. subreniformis; S. subsimplex; S. subsylvaticus; S. suthepensis; S. taiwanensis; S. terrestris; S. thaxteri; S. theobromae; S. thermotolerans; S. variabilis; S. verrucispora; S. verrucosa; S. virgata; S. voglinii; S. waitakere; S. xigazenensis; S. yunnanensis; S. yushuensis; S. zeae; S. zhangmuensis; S. zingiberis; S. zuckii | Stachybotrys chartarum so far known by 132 synonyms (source: MycoBank [29]) | |
| Mycotoxins | MVOCs # | Allergens | Ailments Related with Direct or Indirect Exposure to S. chartarum ^ |
|---|---|---|---|
| triprenylated phenolics; trichodiene; acetone; 2-propanol; 1-propanol; 2-metyl-1-propanol; 1-butanol; 2-butanol; 2-methyl-3-buten-2-ol; 3-methyl-1-butanol; 3-methyl-2-butanol; thujopsene; 2-ethylhexanol; 2-ethylhexyl acetate; methyl benzoate; C15 RI1485 13-farnesene; C15 RI 1513 α-curcumene; C15 RI 1519 β-bisabolene; C15 RI 1544 trichodiene; C15 RI 1545 cuparene; sesquiterpenes; 2-ethylhexanol; 3-methylfuran; dimethylhexadiene; dimethyl disulfide; 1-hexanol; 1-octanol; anisole; 2- and 3-methylanisole; sesquiterpene hydrocarbons; | Sta c 3 (21 kDa protein, 144 aminoacids), extracellular alkaline Mg-dependent exodesoxyribonuclease, IgE inducing; 34 kDa unknown secretory protein (SchS34 open reading frame encodes protein of 221 amino acids in length), localized on surface of conidia; stachyrase A (chymotrypsin-like serine proteinase); aspartyl- and metalloproteases; peroxisomal membrane protein; thioredoxin; glutathione reductase; Mn-superoxide dismutase; cyclophilins; heat shock proteins; enolase; alcohol- and aldehyde dehydrogenases; glycosidases; chitin; glycoproteins; β-1,3-D-glucan; | pulmonary hemorrhage **; gastrointestinal hemorrhage **; sick building syndrome (SBS); mycotoxicosis (stachybotrytoxicosis); leucopenia; lymphadenopathy; agranulocytic anemia; asthma; adult nasal and tracheal bleeding; allergies; inflammation; lung injury; pulmonary hypertension; pulmonary arterial remodeling; irritation and necrotic changes within skin and/or mucous membranes; hypersensitivity pneumonitis (repeated inhalation of conidia); neurotoxicity (induction of apoptosis of olfactory sensory neurons (OSNs) in the olfactory epithelium); inhibitory activity against the complement system (K-76-phenylspirodrimane derivative and its oxidation product, K-76 COOH); headache; fatigue; cough; burning nasal passages; tightness of chest; muscle and stomach aches, |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyląg, M.; Spychała, K.; Zielinski, J.; Łagowski, D.; Gnat, S. Update on Stachybotrys chartarum—Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans. Biology 2022, 11, 352. https://doi.org/10.3390/biology11030352
Dyląg M, Spychała K, Zielinski J, Łagowski D, Gnat S. Update on Stachybotrys chartarum—Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans. Biology. 2022; 11(3):352. https://doi.org/10.3390/biology11030352
Chicago/Turabian StyleDyląg, Mariusz, Klaudyna Spychała, Jessica Zielinski, Dominik Łagowski, and Sebastian Gnat. 2022. "Update on Stachybotrys chartarum—Black Mold Perceived as Toxigenic and Potentially Pathogenic to Humans" Biology 11, no. 3: 352. https://doi.org/10.3390/biology11030352