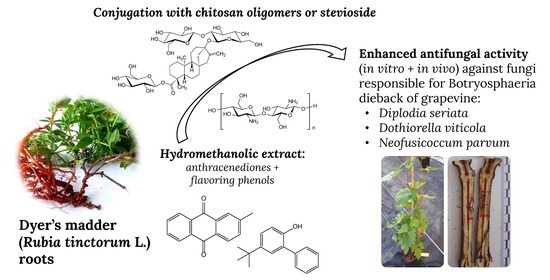
Activity of Anthracenediones and Flavoring Phenols in Hydromethanolic Extracts of Rubia tinctorum against Grapevine Phytopathogenic Fungi
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Chemicals
2.2. Preparation and Physicochemical Characterization of the of R. tinctorum Extracts
2.3. Preparation of Chitosan Oligomers and Bioactive Formulations
2.4. Fungal Isolates
2.5. Antifungal Activity Assessment
2.5.1. In vitro Tests of Mycelial Growth Inhibition
2.5.2. Assays on Autoclaved Grape Wood
2.5.3. Greenhouse Bioassays on Grafted Plants
2.6. Statistical Analyses
3. Results
3.1. Vibrational Characterization
3.2. Gas Chromatography–Mass Spectrometry Analysis of the Extract
3.3. Antifungal Activity
3.3.1. In vitro Tests of Mycelial Growth Inhibition
3.3.2. Assays on Autoclaved Grapevine Wood
3.3.3. Greenhouse Bioassays on Grafted Plants
4. Discussion
4.1. On the Constituents of R. tinctorum Extracts
4.2. On the Combined Effect of Anthraquinones and Phenols
4.3. Comparison with Efficacies Reported in the Literature
4.4. On the Synergistic Behaviour of R. tinctorum Extracts with COS and Stevioside
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Liu, R.; Long, L. Analysis of chemical composition of extractives by acetone and the chromatic aberration of teak (Tectona Grandis L.F.) from China. Molecules 2019, 24, 1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdock, K.C.; Child, R.G.; Fabio, P.F.; Angier, R.D.; Wallace, R.E.; Durr, F.E.; Citarella, R.V. Antitumor agents. 1. 1,4-Bis[(aminoalkyl)amino]-9,10-anthracenediones. J. Med. Chem. 1979, 22, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Coufal, N.; Farnaes, L. Anthracyclines and anthracenediones. In Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures; Minev, B., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 87–102. [Google Scholar] [CrossRef]
- Wuthi-udomlert, M.; Kupittayanant, P.; Gritsanapan, W. In vitro evaluation of antifungal activity of anthraquinone derivatives of Senna alata. J. Health Res. 2010, 24, 117–122. [Google Scholar]
- Agarwal, S.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Singh, D.; Verma, N.; Raghuwanshi, S.; Shukla, P.; Kulshreshtha, D. Antifungal anthraquinones from Saprosma fragrans. Bioorg. Med. Chem. Lett. 2006, 16, 4512–4514. [Google Scholar] [CrossRef]
- Singh, J.; Hussain, Y.; Luqman, S.; Meena, A. Purpurin: A natural anthraquinone with multifaceted pharmacological activities. Phytother. Res. 2020, 35, 2418–2428. [Google Scholar] [CrossRef]
- Essaidi, I.; Snoussi, A.; Koubaier, H.B.H.; Casabianca, H.; Bouzouita, N. Effect of acid hydrolysis on alizarin content, antioxidant and antimicrobial activities of Rubia tinctorum extracts. Pigment. Resin Technol. 2017, 46, 379–384. [Google Scholar] [CrossRef]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-analysis of the core aroma components of grape and wine aroma. Front. Plant Sci. 2016, 7, 1472. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, M.; Kawata, J. Identification of the key aroma compounds in dried roots of Rubia cordifolia. J. Oleo Sci. 2006, 55, 37–39. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-Q.; Quan, M.-P.; Li, Q. Chemical composition and antibacterial activity of the essential oil from Qiancao (Rubia cordifolia Linn.) roots against selected foodborne pathogens. Asian J. Agric. Food Sci. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal activity of eugenol against Botrytis cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, N.; Han, S.S.; Lee, Y.S. Antifungal activity of neutralized wood vinegar with water extracts of Pinus densiflora and Quercus serrata saw dusts. Int. J. Environ. Res. 2009, 3, 167–176. [Google Scholar] [CrossRef]
- Singh, R.; Chauhan, S.M.S.G. 9,10-Anthraquinones and other biologically active compounds from the GenusRubia. Chem. Biodivers. 2004, 1, 1241–1264. [Google Scholar] [CrossRef]
- Angelini, L.G.; Pistelli, L.; Belloni, P.; Bertoli, A.; Panconesi, S. Rubia tinctorum a source of natural dyes: Agronomic evaluation, quantitative analysis of alizarin and industrial assays. Ind. Crop. Prod. 1997, 6, 303–311. [Google Scholar] [CrossRef]
- Boldizsár, I.; Szücs, Z.; Füzfai, Z.; Molnár-Perl, I. Identification and quantification of the constituents of madder root by gas chromatography and high-performance liquid chromatography. J. Chromatogr. A 2006, 1133, 259–274. [Google Scholar] [CrossRef]
- Nakanishi, F.; Nagasawa, Y.; Kabaya, Y.; Sekimoto, H.; Shimomura, K. Characterization of lucidin formation in Rubia tinctorum L. Plant Physiol. Biochem. 2005, 43, 921–928. [Google Scholar] [CrossRef]
- Jalill, R.D.A. GC-MS analysis of extract of Rubia tinctorum having anticancer properties. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 286–292. [Google Scholar]
- Úrbez-Torres, J.R.; Hrycan, J.; Hart, M.; Bowen, P.; Forge, T. Grapevine trunk disease fungi: Their roles as latent pathogens and stress factors that favour disease development and symptom expression. Phytopathol. Mediterr. 2020, 59, 395–424. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magninrobert, M.; Larignon, P.; Chong, J.; Mansour, E.A.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2012, 62, 243–265. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Armengol, J. Fungal Trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine Trunk Diseases: A Review; OIV Publications: Paris, France, 2016; p. 25. [Google Scholar]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langa-Lomba, N.; Buzón-Durán, L.; Sánchez-Hernández, E.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; González-García, V. Antifungal activity against Botryosphaeriaceae fungi of the hydro-methanolic extract of Silybum marianum capitula conjugated with stevioside. Plants 2021, 10, 1363. [Google Scholar] [CrossRef]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of conjugate complexes of chitosan and Urtica dioica or Equisetum arvense extracts for the control of grapevine trunk pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- Nunn, S.; Nishikida, K. Advanced ATR Correction Algorithm—Application Note 50581; ThermoScientific: Madison, WI, USA, 2008; p. 4. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; p. 804. [Google Scholar]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengíbar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and streptomyces spp. secondary metabolites against three botryosphaeriaceae species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytdopathol. Mediterr. 2007, 46, 18–25. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [Green Version]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bull. 1986, 16, 651–657. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Derksen, G.C.H.; Van Beek, T.A. Rubia tinctorum L. In Studies in Natural Products Chemistry; Ur-Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 26, pp. 629–684. [Google Scholar]
- DE Santis, D.; Moresi, M. Production of alizarin extracts from Rubia tinctorum and assessment of their dyeing properties. Ind. Crop. Prod. 2007, 26, 151–162. [Google Scholar] [CrossRef]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural quinone dyes: A review on structure, extraction techniques, analysis and application potential. Waste Biomass Valoriz. 2021, 1–36. [Google Scholar] [CrossRef]
- Derksen, G.; van Beek, T.A.; de Groot, Æ.; Capelle, A. High-performance liquid chromatographic method for the analysis of anthraquinone glycosides and aglycones in madder root (Rubia tinctorum L.). J. Chromatogr. A 1998, 816, 277–281. [Google Scholar] [CrossRef]
- Derksen, G.C.H.; Lelyveld, G.P.; Van Beek, T.A.; Capelle, A. Two validated HPLC methods for the quanti?cation of alizarin and other anthraquinones inRubia tinctorum cultivars. Phytochem. Anal. 2004, 15, 397–406. [Google Scholar] [CrossRef]
- Krizsán, K.; Szókán, G.; Tóth, Z.A.; Hollósy, F.; Laszlo, M.; Khlafulla, A. HPLC Analysis of Anthraquinone Derivatives in Madder Root (Rubia Tinctorum) and Its Cell Cultures. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 2295–2314. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Nafie, M.S.; Khodeer, D.M.; El-Tanahy, A.; Abdel-Kader, M.S.; Badr, J.M.; Abdelhameed, R.F.A. Rubia tinctorum root extracts: Chemical profile and management of type II diabetes mellitus. RSC Adv. 2020, 10, 24159–24168. [Google Scholar] [CrossRef]
- Friedman, M.; Xu, A.; Lee, R.; Nguyen, D.N.; Phan, T.A.; Hamada, S.M.; Panchel, R.; Tam, C.C.; Kim, J.H.; Cheng, L.W.; et al. The inhibitory activity of anthraquinones against pathogenic protozoa, bacteria, and fungi and the relationship to structure. Molecules 2020, 25, 3101. [Google Scholar] [CrossRef]
- Megiatto, J.J.D.; Cazeils, E.; Ham-Pichavant, F.; Grelier, S.; Gardrat, C.; Castellan, A. Styrene-spaced copolymers including anthraquinone and β-O-4 lignin model units: Synthesis, characterization and reactivity under alkaline pulping conditions. Biomacromolecules 2012, 13, 1652–1662. [Google Scholar] [CrossRef]
- Maurino, V.; Borghesi, D.; Vione, D.; Minero, C. Transformation of phenolic compounds upon UVA irradiation of anthraquinone-2-sulfonate. Photochem. Photobiol. Sci. 2007, 7, 321–327. [Google Scholar] [CrossRef]
- Kalyoncu, F.; Çetin, B.; Saglam, H. Antimicrobial activity of common madder (Rubia tinctorum L.). Phytother. Res. 2006, 20, 490–492. [Google Scholar] [CrossRef]
- Manojlovic, N.; Solujic, S.; Sukdolak, S.; Milosev, M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia 2005, 76, 244–246. [Google Scholar] [CrossRef]
- Mehrabian, S.; Majd, A.; Majd, I. Antimicrobial effects of three plants (rubia tinctorum, carthamus tinctorius and juglans regia) on some airborne microorganisms. Aerobiologia 2000, 16, 455–458. [Google Scholar] [CrossRef]
- Ozen, E.; Yeniocak, M.; Goktas, O.; Alma, M.H.; Yilmaz, F. Antimicrobial and antifungal properties of madder root (Rubia tinctorum) colorant used as an environmentally-friendly wood preservative. Bioresources 2014, 9, 1998–2009. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-M.; Lee, C.-H.; Kim, A.H.-G.; Lee, H.-S. Anthraquinones isolated from Cassiatora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. J. Agric. Food Chem. 2004, 52, 6096–6100. [Google Scholar] [CrossRef]
- De Barros, I.B.; Daniel, J.F.D.S.; Pinto, J.P.; Rezende, M.I.; Filho, R.B.; Ferreira, D.T. Phytochemical and antifungal activity of anthraquinones and root and leaf extracts of Coccoloba mollis on phytopathogens. Braz. Arch. Biol. Technol. 2011, 54, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Shang, X.-F.; Zhao, Z.-M.; Li, J.-C.; Yang, G.-Z.; Liu, Y.-Q.; Dai, L.-X.; Zhang, Z.-J.; Yang, Z.-G.; Miao, X.-L.; Yang, C.-J.; et al. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crop. Prod. 2019, 137, 508–520. [Google Scholar] [CrossRef]
- Rath, G.; Ndonzao, M.; Hostettmann, K. Antifungal anthraquinones from Morinda lucida. Int. J. Pharmacogn. 1995, 33, 107–114. [Google Scholar] [CrossRef]
- Mishra, B.B.; Kishore, N.; Tiwari, V.K.; Singh, D.D.; Tripathi, V. A novel antifungal anthraquinone from seeds of Aegle marmelos Correa (family Rutaceae). Fitoterapia 2010, 81, 104–107. [Google Scholar] [CrossRef]
- Tsang, P.W.-K.; Bandara, H.; Fong, W.-P. Purpurin suppresses candida albicans biofilm formation and hyphal development. PLoS ONE 2012, 7, e50866. [Google Scholar] [CrossRef] [Green Version]
- Tsang, P.W.-K.; Wong, A.P.-K.; Yang, H.-P.; Li, N.-F. Purpurin triggers caspase-independent apoptosis in candida dubliniensis biofilms. PLoS ONE 2013, 8, e86032. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.D.O.; Mendes, J.M.; Lima, E.D.O. Investigation on mechanism of antifungal activity of eugenol againstTrichophyton rubrum. Med. Mycol. 2013, 51, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Santamarina, M.P.; Roselló, J.; Giménez, S.; Blázquez, M.A. Commercial Laurus nobilis L. and Syzygium aromaticum L. Merr. & Perry essential oils against post-harvest phytopathogenic fungi on rice. LWT 2016, 65, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Vasconsuelo, A.; Giulietti, A.M.; Boland, R. Signal transduction events mediating chitosan stimulation of anthraquinone synthesis in Rubia tinctorum. Plant Sci. 2004, 166, 405–413. [Google Scholar] [CrossRef]
- Jeevitha, D.; Amarnath, K. Chitosan/PLA nanoparticles as a novel carrier for the delivery of anthraquinone: Synthesis, characterization and in vitro cytotoxicity evaluation. Coll. Surf. B Biointerf. 2013, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.A.; Gazi, M.; Yilmaz, E. Single and binary adsorption of azo and anthraquinone dyes by chitosan-based hydrogel: Selectivity factor and Box-Behnken process design. Chem. Eng. Res. Des. 2015, 104, 264–279. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S. The control of Botrytis fruit rot in strawberry using combined treatments of Chitosan with Zataria multiflora or Cinnamomum zeylanicum essential oil. J. Food Sci. Technol. 2015, 52, 7441–7448. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Ramos-Sánchez, M.D.C.; Pérez-Lebeña, E.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Martín-Ramos, P. Antifungal activity against Fusarium culmorum of stevioside, Silybum marianum seed extracts, and their conjugate complexes. Antibiotics 2020, 9, 440. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Y.; Zhang, T.; Liu, K.; Liu, L.; He, Z.; Xu, B.; Wu, X. Characterization of binding interactions of anthraquinones and bovine β-lactoglobulin. Food Chem. 2019, 281, 28–35. [Google Scholar] [CrossRef]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile preparation of water soluble curcuminoids extracted from turmeric (Curcuma longa L.) powder by using steviol glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.H.; Yu, S.-H.; Kim, J.; An, E.; Hwang, K.; Park, J.-S.; Kim, D. Enhancement of quercetin water solubility with steviol glucosides and the studies of biological properties. Funct. Foods Health Dis. 2015, 5, 437. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the applicability of chitosan oligomers-amino acid conjugate complexes as eco-friendly fungicides against grapevine trunk pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]



| Code | Isolate | Binomial Nomenclature | Geographical Origin | Host/Date |
|---|---|---|---|---|
| ITACYL_F098 | Y-084-01-01a | Diplodia seriata De Not. | Spain (DO Toro) | Grapevine (‘Tempranillo’) 2004 |
| ITACYL_F118 | Y-103-08-01 | Dothiorella viticola A.J.L.Phillips and J.Luque | Spain (Extremadura) | Grapevine 2004 |
| ITACYL_F111 | Y-091-03-01c | Neofusicoccum parvum (Pennycook and Samuels) Crous, Slippers and A.J.L.Phillips | Spain (Navarra, nursery) | Grapevine (‘Verdejo’) 2006 |
| R. tinctorum | Anthraquinone | 4-tert-butyl-2-phenylphenol | Assignment | |
|---|---|---|---|---|
| Root Powder | Extract | |||
| 3334 | 3335 | Bonded O–H stretching (cellulose) | ||
| 2964 | sp3 C–H | |||
| 2920 | 2920 | 2925 | =C–H groups of aromatic rings | |
| 2856 | aliphatic C–H asymmetrical stretching | |||
| 2724 | β–OH, typical of α-hydroxy anthraquinone | |||
| 1727 | 1733 | C=O from esters | ||
| 1704 | ester C=O | |||
| 1676 | C=O in anthraquinones | |||
| 1639 | 1620 | 1633 | C=O in anthraquinones | |
| 1602 | 1605 | 1592 | 1585 | phenyl ring (aromatic skeletal vibration) >C=C< in anthraquinones |
| 1552 | 1545 | carboxylate stretches/C=C aromatic | ||
| 1511 | 1480 | methylene C–H bend | ||
| 1461 | 1470 | methyl C–H asymmetrical | ||
| 1435 | 1430 | =C–H in plane bending | ||
| 1414 | 1416 | 1420 | vinyl C–H in plane bending | |
1370 | 1406 1370 | 1377 1366 | 1385 | C–C asymmetrical stretching phenolic hydroxyl groups |
| 1355 | C–O stretching/methylene C–H bending | |||
| 1333 1329 | 1325 | C–H in-plane deformation methylene C–H bending | ||
| 1316 | 1316 | 1306 | vinylidene C–H in plane bending | |
| 1255 | 1255 | 1287 | 1270 | C–O stretching/C=C symmetric stretching |
| 1207 | 1215 | C–O stretching/C–H in plane bending | ||
| 1171 | 1180 | –C–O–C– stretching | ||
| 1142 | 1153 | 1135 | ||
| 1100 | 1099 | |||
| 1087 | 1080 | |||
| 1020 | 1025 | C–C stretching | ||
| 951 | 969 | C–H out-of-plane bending | ||
| Peak | Rt (min) | Area (%) | Assignments |
|---|---|---|---|
| 1 | 4.6369 | 1.99 | 4-pentenoic acid, ethyl ester |
| 2 | 4.7440 | 0.26 | l-gala-l-ido-octose |
| 3 | 4.8414 | 0.52 | 4-cyclopentene-1,3-dione |
| 4 | 5.0021 | 1.09 | oxime-, methoxy-phenyl- |
| 5 | 5.1968 | 0.46 | 1-(2-furanyl)-ethanone |
| 6 | 5.2942 | 1.36 | 2,5-diethenyltetrahydro-2-methyl-furan |
| 7 | 5.3770 | 1.82 | 2-hydroxy-2-cyclopenten-1-one |
| 8 | 6.0781 | 0.70 | 2,4-dihydroxy-2,5-dimethyl-3(2H)-furan-3-one |
| 9 | 6.3312 | 2.91 | 2-hydroxy-γ-butyrolactone |
| 10 | 6.6331 | 2.47 | glycerin |
| 11 | 6.7694 | 1.93 | 1,2-cyclopentanedione, 3-methyl- |
| 12 | 6.9690 | 1.19 | 2-acetamido-2-deoxy-α-D-glucopyranose |
| 13 | 7.1345 | 0.76 | butyronitrile, 4-ethoxy-3-hydroxy- |
| 14 | 7.2952 | 1.55 | 2,5-dimethyl-4-hydroxy-3(2H)-furanone |
| 15 | 7.4267 | 0.77 | trimethyl(tetrahydrofuran-2-ylperoxy)silane |
| 16 | 7.6798 | 1.01 | 2-methoxy-phenol (or guaiacol) |
| 17 | 7.7869 | 2.20 | L-alanine, methyl ester |
| 18 | 8.2008 | 2.65 | dimethyl dl-malate |
| 19 | 8.3955 | 0.64 | ethanamine, N-ethyl-N-nitroso- |
| 20 | 8.5270 | 2.47 | 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 21 | 9.1453 | 0.87 | 4H-pyran-4-one, 3,5-dihydroxy-2-methyl- |
| 22 | 9.2865 | 0.97 | catechol |
| 23 | 9.4471 | 0.94 | 1,4:3,6-dianhydro-α-d-glucopyranose |
| 24 | 9.6857 | 0.35 | 5-hydroxymethylfurfural |
| 25 | 10.3965 | 0.31 | 2-acetoxy-5-hydroxyacetophenone |
| 26 | 10.5718 | 0.50 | p-cymen-7-ol |
| 27 | 10.8882 | 2.80 | 2-methoxy-4-vinylphenol (or 4-vinylguaiacol) |
| 28 | 11.2193 | 0.98 | DL-arabinose |
| 29 | 11.7451 | 1.09 | DL-proline, 5-oxo-, methyl ester |
| 30 | 12.0470 | 0.74 | vanillin |
| 31 | 12.6702 | 1.96 | 2-methoxy-4-(1-propenyl)-phenol (Z)- (or cis-isoeugenol) |
| 32 | 13.1473 | 1.05 | 1-[4-(methylthio)phenyl]-ethanone |
| 33 | 13.4492 | 0.86 | butylated hydroxytoluene |
| 34 | 13.6877 | 0.57 | benzeneacetic acid, 4-hydroxy-3-methoxy-, methyl ester |
| 35 | 13.9458 | 0.82 | 1,4-diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol |
| 36 | 14.3693 | 2.44 | α-methyl-l-sorboside |
| 37 | 15.0461 | 5.78 | guanosine |
| 38 | 15.5865 | 8.31 | 1,4-diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol |
| 39 | 16.0734 | 1.65 | 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol (or coniferyl alcohol) |
| 40 | 17.4561 | 0.43 | 5-amino-1-(4-amino-furazan-3-yl)-1H-[1,2,3]triazole-4-carbonitrile |
| 41 | 17.9088 | 1.18 | hexadecanoic acid, methyl ester |
| 42 | 18.2594 | 1.17 | n-hexadecanoic acid |
| 43 | 19.1552 | 0.76 | 5-(1,1-dimethylethyl)[1,1′-biphenyl]-2-ol (or 4-tert-butyl-2-phenylphenol) |
| 45 | 19.4278 | 0.69 | cyclopentadecane |
| 46 | 19.6421 | 0.15 | 1-hydroxy-9,10-anthracenedione (or α-hydroxyanthraquinone) |
| 47 | 19.8709 | 15.54 | 9,10-anthracenedione, 2-methyl- (or β-methylanthraquinone) |
| 48 | 20.7278 | 1.43 | 1-hydroxy-4-methylanthraquinone |
| 49 | 21.1659 | 1.75 | 1,2-dihydroxyanthraquinone (or alizarin) |
| 50 | 21.8038 | 1.40 | azacyclotridecan-2-one, 1-(3-aminopropyl)- |
| 51 | 23.0550 | 0.81 | glycerol 1-palmitate |
| 52 | 23.3812 | 0.73 | bis(2-ethylhexyl) phthalate |
| 53 | 23.8973 | 0.24 | 1,8-dihydroxy-3-methyl-9,10-anthracenedione (or 1,8-dihydroxy-3-methyl anthraquinone) |
| 54 | 24.2673 | 8.57 | 4-methoxy-4′,5′-methylenedioxybiphenyl-2-carboxylic acid |
| 55 | 24.4474 | 0.42 | 9-octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 56 | 25.4260 | 0.35 | squalene |
| 57 | 29.2333 | 0.62 | octasiloxane, 1,1,3,3,5,5,7,7,9,9,11,11,13,13,15,15-hexadecamethyl- |
| 58 | 29.9051 | 0.40 | γ-sitosterol |
| Pathogen | EC | COS | Stevioside | R. tinctorum | COS— R. tinctorum | Stevioside —R. tinctorum | 4-tert… | 1,2,4-trihydro… | Guanosine |
|---|---|---|---|---|---|---|---|---|---|
| D. seriata | EC50 | 744.4 ± 43.9 | 288.1 ± 15.3 | 78.0 ± 0.8 | 63.1 ± 0.3 | 73.6 ± 0.3 | 53.0 ± 2.1 | 45.4 ± 3.4 | 130.4 ± 12.8 |
| EC90 | 1179.9 ± 58.2 | 840.5 ± 62.3 | 87.8 ± 1.9 | 73.4 ± 0.9 | 82.4 ±0.7 | 73.2 ± 2.3 | 171.4 ± 18.7 | 249.9 ± 28.5 | |
| D. viticola | EC50 | 554.3 ± 27.4 | 306.9 ± 26.6 | 66.2 ± 2.9 | 22.1 ± 1.4 | 80.0 ± 0.7 | 25.7 ± 3.6 | 37.2 * | 182.7 ± 7.7 |
| EC90 | 1138.7 ± 75.0 | 917.0 ± 74.3 | 90.2 ± 8.7 | 55.5 ± 4.6 | 90.7 ± 1.5 | 71.2 ± 9.0 | 74.9 * | 308.1 ± 23.7 | |
| N. parvum | EC50 | 680.2 ± 43.1 | 194.8 ± 13.4 | 92.3 ± 0.5 | 38.2 ± 1.4 | 75.1 ± 0.8 | 62.2 ± 0.7 | 72.0 ± 14.8 | 95.1 ± 22.6 |
| EC90 | 1326.6 ± 83.2 | 723.8 ± 56.7 | 184.0 ± 1.1 | 66.3 ± 4.2 | 89.2 ± 1.9 | 70.6 ± 2.2 | 338.4 ± 37.9 | 317.8 ± 33.9 |
| Pathogen | EC | Synergy Factor | |
|---|---|---|---|
| COS-R. tinctorum | Stevioside—R. tinctorum | ||
| D. seriata | EC50 | 2.24 | 1.67 |
| EC90 | 2.23 | 1.93 | |
| D. viticola | EC50 | 5.35 | 1.36 |
| EC90 | 3.01 | 1.81 | |
| N. parvum | EC50 | 4.26 | 1.67 |
| EC90 | 4.87 | 3.29 | |
| Sample | Frequency | Sum of Ranks | Mean of Ranks | Groups | ||
|---|---|---|---|---|---|---|
| COS-R. tinctorum negative control | 24 | 300.000 | 12.500 | A | ||
| COS-R. tinctorum-D. seriata | 120 | 10,193.000 | 84.942 | B | ||
| Positive control | 24 | 3703.000 | 154.292 | C | ||
| Pathogen | Sample | Frequency | Sum of Ranks | Mean of Ranks | Groups | ||
|---|---|---|---|---|---|---|---|
| D. seriata | COS-R. tinctorum negative control | 32 | 725.500 | 22.672 | A | ||
| COS-R. tinctorum-D. seriata | 72 | 6124.000 | 85.056 | B | |||
| Positive control | 56 | 6030.500 | 107.688 | C | |||
| D. viticola | COS-R. tinctorum negative control | 32 | 1295.000 | 40.469 | A | ||
| COS-R. tinctorum-D. viticola | 72 | 4885.000 | 67.847 | B | |||
| Positive control | 64 | 8016.000 | 125.250 | C | |||
| N. parvum | COS-R. tinctorum negative control | 32 | 572.000 | 17.875 | A | ||
| COS-R. tinctorum-N. parvum | 48 | 3695.000 | 76.979 | B | |||
| Positive control | 64 | 6173.000 | 96.453 | C | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langa-Lomba, N.; Sánchez-Hernández, E.; Buzón-Durán, L.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Activity of Anthracenediones and Flavoring Phenols in Hydromethanolic Extracts of Rubia tinctorum against Grapevine Phytopathogenic Fungi. Plants 2021, 10, 1527. https://doi.org/10.3390/plants10081527
Langa-Lomba N, Sánchez-Hernández E, Buzón-Durán L, González-García V, Casanova-Gascón J, Martín-Gil J, Martín-Ramos P. Activity of Anthracenediones and Flavoring Phenols in Hydromethanolic Extracts of Rubia tinctorum against Grapevine Phytopathogenic Fungi. Plants. 2021; 10(8):1527. https://doi.org/10.3390/plants10081527
Chicago/Turabian StyleLanga-Lomba, Natalia, Eva Sánchez-Hernández, Laura Buzón-Durán, Vicente González-García, José Casanova-Gascón, Jesús Martín-Gil, and Pablo Martín-Ramos. 2021. "Activity of Anthracenediones and Flavoring Phenols in Hydromethanolic Extracts of Rubia tinctorum against Grapevine Phytopathogenic Fungi" Plants 10, no. 8: 1527. https://doi.org/10.3390/plants10081527