The Alien Plant Species Impact in Rice Crops in Northwestern Italy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Flora
3.2. Vegetation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Gentili, R.; Schaffner, U.; Martinoli, A.; Citterio, S. Invasive alien species and biodiversity: Impacts and management. Biodiversity 2021, 22, 1–3. [Google Scholar] [CrossRef]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Gallardo, B.; Bacher, S.; Bradley, B.; Comín, F.; Gallien, L.; Jeschke, J.; Sorte, C.; Vilà, M. InvasiBES: Understanding and managing the impacts of Invasive alien species on Biodiversity and Ecosystem Services. NeoBiota 2019, 50, 109–122. [Google Scholar] [CrossRef]
- Seabloom, E.W.; Williams, J.W.; Slayback, D.; Stoms, D.M.; Viers, J.H.; Dobson, A.P. Human impacts, plant invasion, and imperiled plant species in California. Ecol. Appl. 2006, 16, 1338–1350. [Google Scholar] [CrossRef]
- Baker, R.; Cannon, R.; Bartlett, P.; Barker, I. Novel strategies for assessing and managing the risks posed by alien species to global crop production and biodiversity. Ann. Appl. Biol. 2005, 146, 177–191. [Google Scholar] [CrossRef]
- Mack, R.; Simberloff, D.; Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Walker, B.; Steffen, W. An Overview of the Implications of Global Change for Natural and Managed Terrestrial Ecosystems. Conserv. Ecol. 1996, 1, 2. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Assini, S.; Banfi, E.; Barni, E.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; et al. Non-native flora of Italy: Species distribution and threats. Plant Biosyst. 2010, 144, 12–28. [Google Scholar] [CrossRef]
- Dogra, K.; Sood, S.; Dobhal, P.; Sharma, S. Alien plant invasion and their impact on indigenous species diversity at global scale: A review. J. Ecol. Nat. Environ. 2010, 2, 175–186. [Google Scholar]
- Clout, M.; De Poorter, M. IUCN Guidelines for the Prevention Of Biodiversity Loss Caused By Alien Invasive Species. Aliens 2000, 11, 1–21. [Google Scholar]
- IUCN. Invasive Alien Species and Climate Change. Available online: https://www.iucn.org/sites/default/files/2022-04/ias_and_climate_change_issues_brief_2021.pdf (accessed on 16 May 2023).
- IUCN. Unified Classification of Direct Threats: Version 3.2. Available online: https://nc.iucnredlist.org/redlist/content/attachment_file/dec_2012_guidance_threats_classification_scheme.pdf (accessed on 13 March 2023).
- Convention on Biological Diversity. Available online: https://www.cbd.int/invasive/ (accessed on 14 March 2023).
- Secretariat of the Convention on Biological Diversity. Invasive Alien Species, A Threat To Biodiversity; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009.
- Levine, J.M.; Vilà, M.; Antonio, C.M.D.; Dukes, J.S.; Grigulis, K.; Lavorel, S. Mechanisms underlying the impacts of exotic plant invasions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 775–781. [Google Scholar] [CrossRef]
- Bai, B.B. Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species. Environ. Entomol. 2008, 37, 277. [Google Scholar] [CrossRef]
- Inghilesi, A.F.; Mazza, G.; Cervo, R.; Gherardi, F.; Sposimo, P.; Tricarico, E.; Zapparoli, M. Alien insects in Italy: Comparing patterns from the regional to European level. J. Insect Sci. 2013, 13, 73. [Google Scholar] [CrossRef]
- Adla, K.; Dejan, K.; Neira, D.; Dragana, Š. Chapter 9—Degradation of ecosystems and loss of ecosystem services. In One Health; Prata, J.C., Ribeiro, A.I., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 281–327. [Google Scholar]
- Markandya, A. The Economic Feedbacks of Loss of Biodiversity and Ecosystems Services. OECD Environ. Work. Pap. 2015, 93, 1–26. [Google Scholar] [CrossRef]
- Domina, G. Invasive Aliens in Italy. In Invasive Alien Species: Observations and Issues from Around the World, Volume 3: Issues and Invasions in Europe, First Edition; John Wiley & Sons: New York, NY, USA, 2021; pp. 190–214. [Google Scholar]
- Galasso, G.; Domina, G.; Adorni, M.; Ardenghi, N.; Bonari, G.; Buono, S.; Cancellieri, L.; Chianese, G.; Ferretti, G.; Fiaschi, T.; et al. Notulae to the Italian alien vascular flora: 5. Ital. Bot. 2018, 5, 45–56. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Alessandrini, A.; Ardenghi, N.; Bacchetta, G.; Ballelli, S.; Bartolucci, F.; Brundu, G.; Buono, S.; Busnardo, G.; et al. Notulae to the Italian alien vascular flora: 6. Ital. Bot. 2018, 6, 65–90. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Ardenghi, N.; Aristarchi, C.; Bacchetta, G.; Bartolucci, F.; Bonari, G.; Bouvet, D.; Brundu, G.; Buono, S.; et al. Notulae to the Italian alien vascular flora: 7. Ital. Bot. 2019, 7, 157–182. [Google Scholar] [CrossRef]
- Galasso, G.; Domina, G.; Andreatta, S.; Angiolini, C.; Ardenghi, N.; Aristarchi, C.; Arnoul, M.; Azzella, M.; Bacchetta, G.; Bartolucci, F.; et al. Notulae to the Italian alien vascular flora: 8. Ital. Bot. 2019, 8, 63–93. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Viciani, D.; Vidali, M.; Gigante, D.; Bolpagni, R.; Villani, M.; Acosta, A.; Adorni, M.; Aleffi, M.; Allegrezza, M.; Angiolini, C.; et al. A first checklist of the alien-dominated vegetation in Italy. Plant Sociol. 2020, 57, 29–54. [Google Scholar] [CrossRef]
- Lazzaro, L.; Bolpagni, R.; Buffa, G.; Gentili, R.; Lonati, M.; Stinca, A.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; et al. Impact of invasive alien plants on native plant communities and Natura 2000 habitats: State of the art, gap analysis and perspectives in Italy. J. Environ. Manag. 2020, 274, 111140. [Google Scholar] [CrossRef]
- Pellegrini, E.; Buccheri, M.; Martini, F.; Boscutti, F. Agricultural land use curbs exotic invasion but sustains native plant diversity at intermediate levels. Sci. Rep. 2021, 11, 8385. [Google Scholar] [CrossRef]
- Cossu, T.A.; Camarda, I.; Brundu, G. A catalogue of non-native weeds in irrigated crops in Sardinia (Italy). Webbia 2014, 69, 145–156. [Google Scholar] [CrossRef]
- Viggiani, P.; Tabacchi, M. Piante Infestanti di Risaie e Canali: Botanica e Riconoscimento; Edagricole: Milano, Italy, 2017. [Google Scholar]
- Viggiani, P. Weed flora in Italian rice fields. In Proceedings of the 13th EWRS symposium, Bari, Italy, 19–23 June 2005. [Google Scholar]
- European Commission. agridata.ec.europa.eu. Available online: https://agridata.ec.europa.eu/extensions/DashboardRice/RiceProduction.html (accessed on 1 March 2023).
- Pignatti, S. La vegetazione delle risaie pavesi: Studio fitosociologico. Arch. Bot. Biogeogr. Ital. 1957, 33, 129–193. [Google Scholar]
- Tomaselli, R. Aspetti della vegetazione in risaia da vicenda del Pavese e del Vercellese prima e dopo il diserbo. Arch. Bot. Biogeogra. Ital. 1958, 34, 217–253. [Google Scholar]
- Piccoli, F.; Gerdol, R. Rice-field weed communities in Ferrara Province (northern Italy). Aquat. Bot. 1981, 10, 317–328. [Google Scholar] [CrossRef]
- Covarelli, G. Evoluzione della flora e della vegetazione infestante le principali colture agrarie in Italia. Fitosociologia 2002, 39, 3–13. [Google Scholar]
- Ferrero, A.; Tinarelli, A. Rice Cultivation in the E.U. Ecological Conditions and Agronomical Practices. In Pesticide Risk Assessment in Rice Paddies: Theory and Practice; Capri, E., Karpouzas, D.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hill, J.H.; Bayer, D.E.; Bocchi, S.; Clampett, W.S. Direct Seeded Rice in the Temperate Climates of Australia, Italy and North America; International Rice Research Institute: Los Baños, Pilippines, 1991; Volume IRRI Monograph, pp. 91–103. [Google Scholar]
- Monaco, S.; Volante, A.; Orasen, G.; Cochrane, N.; Oliver, V.; Price, A.H.; Teh, Y.A.; Martínez-Eixarch, M.; Thomas, C.; Courtois, B.; et al. Effects of the application of a moderate alternate wetting and drying technique on the performance of different European varieties in Northern Italy rice system. Field Crop. Res. 2021, 270, 108220. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Manfron, G.; Toreti, A.; Duveiller, G.; Romani, M.; Rocca, C.; Scoccimarro, E.; Podrascanin, Z.; Djurdjevic, V. Adaptation and sustainability of water management for rice agriculture in temperate regions: The Italian case-study. Land Degrad. Dev. 2019, 30, 2033–2047. [Google Scholar] [CrossRef]
- Rossi, G.; Tazzari, E.; Abeli, T.; Cauzzi, P.; Ardenghi, N.; Orsenigo, S.; Vagge, I. Activities and Perspectives of Plant Diversity Conservation in Rice Paddies (and Surrounding) of Po River Plain, N-Italy. In Organic Rice Farming and Production Systems: Conference Folder; Università degli Studi di Milano: Milan, Italy, 2015. [Google Scholar]
- Fasola, M.; Ruiz, X. The Value of Rice Fields as Substitutes for Natural Wetlands for Waterbirds in the Mediterranean Region. Colon. Waterbirds 1996, 19, 122. [Google Scholar] [CrossRef]
- Sánchez-Guzmán, J.M.; Morán, R.; Masero, J.A.; Corbacho, C.; Costillo, E.; Villegas, A.; Santiago-Quesada, F. Identifying new buffer areas for conserving waterbirds in the Mediterranean basin: The importance of the rice fields in Extremadura, Spain. Biodivers. Conserv. 2007, 16, 3333–3344. [Google Scholar] [CrossRef]
- Bogliani, G.; Della Rocca, F. Biodiversity and Rice Production in Rice Agro-Ecosystem—The Action Plan—Action E.5; International Rice Field Ecological Network LIFE Project LIFE09 NAT/IT/000093 ECORICE; International Rice Research Institute: Manila, Philippines, 2014. [Google Scholar]
- Orsenigo, S.; Corli, A. Buone Pratiche di Gestione di Risaie e Prati Umidi per la Conservazione di Specie Vegetali di Interesse Comunitario; Università di Pavia: Pavia, Italy, 2022; Volume Manuali del progetto CLOVER: Agroecosistemi e Conservazione in LOmbardia di specie VEgetali Rare di Direttiva Habitat. [Google Scholar]
- Corli, A.; Orsenigo, S.; Gerdol, R.; Bocchi, S.; Smolders, A.P.; Brancaleoni, L.; Caffi, M.T.; Abeli, T.; Rossi, G. Coexistence of rice production and threatened plant species: Testing Marsilea quadrifolia L. in N-Italy. Paddy Water Environ. 2021, 19, 395–400. [Google Scholar] [CrossRef]
- Vagge, I.; Chiaffarelli, G. Validating the Contribution of Nature-Based Farming Solutions (NBFS) to Agrobiodiversity Values through a Multi-Scale Landscape Approach. Agronomy 2023, 13, 233. [Google Scholar] [CrossRef]
- Dramstad, W.E.; Olson, J.D.; Forman, R.T.T. Landscape Ecology Principles in Landscape Architecture and Land Use Planning; Island Press: Washington, DC, USA, 1996. [Google Scholar]
- Dover, J.W.; Bunce, R.G.H. Key Concepts in Landscape Ecology; IALE UK, Coplin Cross Printers Ltd.: Garstang, UK, 1998. [Google Scholar]
- Forman, R.T.T. Land Mosaics: The Ecology of Landscapes and Regions, 1st ed.; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Forman, R.T.T.; Godron, M. Landscape Ecology; J. Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Turner, M.G.; Gardner, R.H. Landscape Ecology in Theory and Practice, Pattern and Process; Springer Verlag: New York, NY, USA, 2015. [Google Scholar]
- Ingegnoli, V. Landscape Bionomics: Biological-Integrated Lanscape Ecology; Springer: Milan, Italy, 2015. [Google Scholar]
- Ingegnoli, V. Landscape Ecology: A Widening Foundation; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the worldwide bioclimatic classification system. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Globalbioclimatics. Available online: www.globalbioclimatics.org (accessed on 21 October 2022).
- Rivas-Martínez, S. Global Bioclimatics, Clasificación Bioclimática de la Tierra; CIF: Madrid, Spain, 2004. [Google Scholar]
- Rivas-Martínez, S.; Sáenz, S.; Penas, A. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 634. [Google Scholar]
- Geoportale Piemonte. Available online: www.geoportale.piemonte.it/cms/ (accessed on 10 October 2022).
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2017–2019. [Google Scholar]
- Dryades. Available online: http://dryades.units.it/cercapiante/index.php (accessed on 11 October 2022).
- Flora Italiae ActaPlantarum. Available online: www.floraitaliae.actaplantarum.org (accessed on 11 October 2022).
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon press: Oxford, UK, 1934. [Google Scholar]
- Domina, G.; Galasso, G.; Bartolucci, F.; Guarino, R. Ellenberg Indicator Values for the vascular flora alien to Italy. Electronic Supplementary File 1. Flora Mediterr. 2018, 28, 53–61. [Google Scholar] [CrossRef]
- Guarino, R.; Domina, G.; Pignatti, S. Ellenberg’s Indicator values for the Flora of Italy—First update: Pteridophyta, Gymnospermae and Monocotyledoneae. Flora Mediterr. 2012, 22, 197–209. [Google Scholar] [CrossRef]
- Pignatti, S.; Menegoni, P.; Pietrosanti, S. Valori di bioindicazione delle piante vascolari della flora d’Italia, Bioindicator values of vascular plants of the flora of Italy. Braun-Blanquetia Rev. Geobot. Monogr. 2005, 39, 1–97. [Google Scholar]
- Pomini, L. Saggio di Flora Della Risaia Vercellese-Novarese; Istituto Tecnico Agrario di Vercelli—S.A.V.I.T.: Vercelli, Italy, 1957. [Google Scholar]
- Pomini, L. Piante Vascolari Infestanti la Risaia; Istituto Tecnico Agrario di Vercelli: Vercelli, Italy, 1955; Volume 3. [Google Scholar]
- Ciferri, R.; Giacomini, V.; Poggio, P. La Flora Fanerogamica Delle Risaie Dell’italia Transpadana. Suppl. Agli Atti Dell’istituto Bot. Dell’università Lab. Crittogam. Pavia 1949, 5, 1–26. [Google Scholar]
- Koch, W. Pflanzensoziologische skizzen aus den reisfeld-gebieten des piemont (Po-ebene). Vegetatio 1954, 5/6, 487–493. [Google Scholar] [CrossRef]
- Koch, W. Zur Flora der Oberitalienischen Reisfelder; Buchdruckerei Buchler & Co: Bern, Switzerland, 1952; Volume 62. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Wien, Austria; New York, NY, USA, 1964. [Google Scholar]
- Géhu, J.M. L’analyse symphytosociologique et géosymphytosociologique de l’espace, Théorie et métodologie. Coll. Phytosoc. 1988, XVII, 11–46. [Google Scholar]
- Géhu, J.M.; Rivas-Martínez, S. Notions fondamentales de phytosociologie. Ber. Int. Simp. Int. Ver. Veg. 1981, 31.3, 5–33. [Google Scholar]
- Pirola, A. Elementi di Fitosociologia; CLUEB: Bologna, Italy, 1970. [Google Scholar]
- Rivas-Martínez, S. Nociones sobre Fitosociología, Biogeografía e Bioclimatología. In La Vegetation de España; Universidad de Alcalá de Henares: Madrid, Spain, 1987; pp. 19–45. [Google Scholar]
- van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Berlin/Heidelberg, Germany, 1928. [Google Scholar]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Vagge, I.; Blasi, C. New and validated syntaxa for the checklist of Italian vegetation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 318–332. [Google Scholar] [CrossRef]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Soriano, P.; Tesei, G.; Blasi, C. New insight on Mediterranean and sub-Mediterranean syntaxa included in the Vegetation Prodrome of Italy. Flora Mediterr. 2015, 25, 77–102. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia, S.; Pesaresi, S.; Gangale, C.; Uzunov, D. New syntaxa for the prodrome of Italian vegetation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 723–727. [Google Scholar] [CrossRef]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Poldini, L.; Sburlino, G.; Vagge, I.; Venanzoni, R. New syntaxonomic contribution to the Vegetation Prodrome of Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 149, 603–615. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 728–814. [Google Scholar] [CrossRef]
- Geoportale Nazionale. Available online: www.pcn.minambiente.it/mattm/ (accessed on 10 October 2022).
- Prodromo Della Vegetazione Italiana. Available online: www.prodromo-vegetazione-italia.org (accessed on 21 October 2022).
- Verde, S.; Assini, S.; Andreis, C. Le Serie di Vegetazione Della Regione Lombardia. Available online: https://air.unimi.it/handle/2434/149481 (accessed on 16 May 2023).
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–125. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Ferrero, A.; Vidotto, F. Weeds and weed management in Italian rice fields. In Agro-Economical Traits of Rice Cultivation in Europe and India; Edizioni Mercurio: Vercelli, Italy, 2007; pp. 55–72. [Google Scholar]
- Rossi, G.; Montagnani, C.; Gargano, D.; Peruzzi, L.; Abeli, T.; Ravera, S.; Cogoni, A.; Giuseppe, F.; Magrini, S.; Gennai, M.; et al. Lista Rossa della Flora Italiana. 1. Policy Species e Altre Specie Minacciate; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Rome, Italy, 2013. [Google Scholar]
- Rossi, G.; Orsenigo, S.; Gargano, D.; Montagnani, C.; Peruzzi, L.; Giuseppe, F.; Abeli, T.; Alessandrini, A.; Astuti, G.; Bacchetta, G.; et al. Lista Rossa IUCN Della Flora Italiana: 2. ENDEMITI e Altre Piante Minacciate; Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Rome, Italy, 2020. [Google Scholar]
- Ferrero, A.; Tesio, F.; Tabacchi, M.; Vidotto, F. The effects of water management, timing and the rate of several herbicides on the growth of Murdannia keisak (Hassk.) Handel-Mazz. Crop Prot. 2012, 38, 53–56. [Google Scholar] [CrossRef]
- O’Reilly-Nugent, A.; Palit, R.; Lopez-Aldana, A.; Medina-Romero, M.; Wandrag, E.; Duncan, R.P. Landscape Effects on the Spread of Invasive Species. Curr. Landsc. Ecol. Rep. 2016, 1, 107–114. [Google Scholar] [CrossRef]
- Carretero, J.L. La vegetacion emergente de los arrozales europeos. Anales de Biologia 1989, 15, 135–141. [Google Scholar]
- European Parliament. Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the conservation of wild birds. Off. J. Eur. Union 2010, L 20/7, 19. [Google Scholar]
- Council of the European Communities. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 50. [Google Scholar]
- Chiatante, G.; Pellitteri-Rosa, D.; Torretta, E.; Nonnis Marzano, F.; Meriggi, A. Indicators of biodiversity in an intensively cultivated and heavily human modified landscape. Ecol. Indic. 2021, 130, 108060. [Google Scholar] [CrossRef]
- Maskell, L.C.; Botham, M.; Henrys, P.; Jarvis, S.; Maxwell, D.; Robinson, D.A.; Rowland, C.S.; Siriwardena, G.; Smart, S.; Skates, J.; et al. Exploring relationships between land use intensity, habitat heterogeneity and biodiversity to identify and monitor areas of High Nature Value farming. Biol. Conserv. 2019, 231, 30–38. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Ali, M.P.; Biswas, M.; Clemente-Orta, G.; Kabir, M.M.M.; Datta, J.; Haque, S.S.; Qin, X.; Landis, D.; Kaur, P.; Pittendrigh, B.R.; et al. Landscape diversity influences the arthropod species diversity in the rice field. Front. Environ. Sci. 2022, 10, 15. [Google Scholar] [CrossRef]
- Holland, J.; Fahrig, L. Effect of woody borders on insect density and diversity in crop fields: A landscape-scale analysis. Agric. Ecosyst. Environ. 2000, 78, 115–122. [Google Scholar] [CrossRef]
- Smart, S.M.; Marrs, R.H.; Le Duc, M.G.; Thompson, K.E.N.; Bunce, R.G.H.; Firbank, L.G.; Rossall, M.J. Spatial relationships between intensive land cover and residual plant species diversity in temperate farmed landscapes. J. Appl. Ecol. 2006, 43, 1128–1137. [Google Scholar] [CrossRef]
- Schindler, S.; von Wehrden, H.; Poirazidis, K.; Wrbka, T.; Kati, V. Multiscale performance of landscape metrics as indicators of species richness of plants, insects and vertebrates. Ecol. Indic. 2013, 31, 41–48. [Google Scholar] [CrossRef]









| SITE 1 (G) | SITES 2 (D)−3 (P) | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8G | 4G | 9G | 14G | 11G | 15G | 12G | 10G | 13G | 2G | 1G | 3G | 7G | 5G | 6G | 20D | 22D | 26P | 28P | 29P | 24D | 39P | 25P | 40P | 37P | 36P | 38P | 23D | 31P | 33P | 21D | 32P | 16D | 17D | 18D | 19D | 27P | 34P | 30P | 35P | ||||
| Coverage (%) | 95 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 95 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | pres. | freq. | |
| Area (m2) | 70 | 80 | 70 | 85 | 100 | 90 | 90 | 75 | 90 | 90 | 90 | 80 | 70 | 60 | 80 | 100 | 100 | 80 | 90 | 90 | 100 | 100 | 90 | 100 | 100 | 90 | 95 | 100 | 95 | 85 | 100 | 90 | 100 | 100 | 100 | 100 | 80 | 90 | 90 | 100 | |||
| Species number | 8 | 10 | 7 | 10 | 7 | 10 | 9 | 10 | 10 | 7 | 9 | 12 | 8 | 8 | 9 | 9 | 9 | 8 | 9 | 11 | 5 | 4 | 9 | 7 | 4 | 5 | 4 | 3 | 8 | 7 | 7 | 9 | 11 | 14 | 10 | 10 | 10 | 9 | 7 | 8 | |||
| Os Oryza sativa s.l. | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 4.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 4.4 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 4.4 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 40 | V | |
| Char. and diff. species of Oryzo sativae−Echinocletum cruris galli | |||||||||||||||||||||||||||||||||||||||||||
| Ec Echinocloa crus−galli (L.) P. Beauv. | 1.1 | + | 1.2 | +0.2 | 1.2 | + | − | +0.2 | 1.1 | + | 1.2 | + | +0.2 | 1.2 | − | − | 2.3 | 2.2 | 1.2 | + | 1.1 | + | 1.2 | + | + | + | − | − | +0.2 | + | + | + | − | +0.2 | +0.2 | 1.2 | + | + | 1.2 | + | 34 | V | |
| Ld Lindernia dubia (L.) Pennell | − | − | − | − | − | − | + | + | + | − | + | − | − | 1.1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2.2 | + | + | + | − | − | − | − | 10 | II | |
| Char. and diff. species of the upper units (Oryzo−Echinochloion oryzoidis alliance, Cypero−Echinochloetalia oryzoidis order, Oryzetea sativae class) | |||||||||||||||||||||||||||||||||||||||||||
| Sm | Schoenoplectiella mucronata (L.) J. Jung & H.K. Choi | 2.3 | 3.4 | 2.3 | 1.2 | 1.2 | 2.2 | 1.1 | 1.2 | 1.1 | 3.3 | +.2 | 2.3 | 3.3 | 2.3 | 3.4 | + | 1.2 | 3.3 | + | +0.2 | − | − | 2.2 | − | − | 1.1 | + | − | + | + | − | +0.2 | + | 1.1 | − | + | +.2 | 1.2 | 1.1 | 2.2 | 33 | IV |
| Hr | Heteranthera reniformis Ruiz & Pav. | − | 1.2 | − | 1.2 | 3.3 | 2.2 | 2.3 | 2.4 | 3.3 | 1.1 | + | 2.3 | − | − | − | − | − | − | − | + | − | − | 1.1 | − | − | − | − | − | + | − | 3.3 | + | − | + | + | + | 2.3 | 2.3 | 1.2 | 1.3 | 22 | III |
| Av | Ammania verticillata (Ard.) Lam. | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | 1.1 | − | + | 1.2 | + | − | + | 3.3 | 2.3 | 1.2 | 2.2 | 4.4 | 1.2 | 2.3 | 2.2 | 2.3 | 2.2 | 3.3 | 20 | III |
| Ep | Eleocharis flavescens (Poir.) Urb. | +0.2 | 1.2 | + | +0.2 | 1.3 | 1.2 | − | +0.2 | 1.2 | 1.1 | +0.2 | 1.2 | 2.3 | 3.4 | 2.4 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 14 | II |
| Hl | Heteranthera limosa (Sw.) Willd. | + | + | − | +0.2 | − | + | 1.2 | 1.3 | − | − | − | 3.4 | 2.3 | 3.4 | 1.2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 10 | II |
| Mk | Murdannia keisak (Hassk.) Hand.−Mazz. | − | + | − | 1.1 | − | + | − | + | − | +0.2 | + | 3.4 | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 8 | I |
| Rd | Rotala densiflora (Roth) Koehne | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | − | + | 1.1 | − | + | + | 1.2 | + | 1.2 | − | 1.1 | 1.1 | 2.3 | 13 | II |
| Ea | Eleocharis acicularis (L.) Roem. & Schult. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | +.2 | − | − | − | − | − | − | − | − | − | − | 1.2 | + | 1.2 | 1.2 | − | − | − | + | − | − | − | − | − | 7 | I |
| Oss | Oryza sativa L. var. sylvatica Chiappelli | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1.2 | 2.2 | − | − | +0.2 | − | − | − | − | − | − | − | − | − | − | +0.2 | − | − | +0.2 | − | − | − | 5 | I |
| Others species | |||||||||||||||||||||||||||||||||||||||||||
| Pl | Persicaria lapathifolia (L.) Delarbre | 2.3 | + | 1.1 | − | 2.2 | − | 1.1 | + | +0.2 | + | 2.3 | 2.2 | 1.2 | + | 2.3 | 1.1 | − | 1.1 | − | + | − | + | 1.2 | + | − | − | − | − | − | 1.2 | − | 1.2 | − | − | + | − | + | − | − | − | 23 | III |
| Ap | Alisma plantago−aquatica L. | 1.1 | − | − | − | 1.1 | + | + | 1.2 | 1.1 | − | +0.2 | 1.2 | − | − | − | − | − | 1.1 | + | +0.2 | − | − | + | − | − | − | − | − | − | − | 1.2 | + | 4.4 | 2.3 | 2.2 | 1.2 | +0.2 | + | +0.2 | − | 21 | III |
| Bf | Bidens frondosa L. | + | + | − | + | − | + | + | − | + | − | − | − | − | − | + | + | + | + | 2.2 | − | − | + | − | + | − | − | − | − | − | − | − | − | + | + | + | 1.2 | − | − | − | + | 18 | III |
| Bu | Butomus umbellatus L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1.2 | 3.2 | − | 1.2 | 1.2 | − | − | − | − | − | − | − | + | + | − | +0.2 | 1.2 | 1.1 | + | − | + | − | − | − | − | 11 | II |
| Lm | Lemna minor L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | +0.2 | 2.3 | − | − | +0.3 | 1.1 | − | − | − | − | − | − | − | − | + | − | − | − | + | 1.1 | − | + | 8 | I |
| Ta | Typha angustifolia L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1.2 | + | − | − | + | + | − | − | 4 | I |
| Jc | Juncus conglomeratus L. | − | − | − | − | − | − | − | − | 1.2 | − | − | 1.1 | 1.1 | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 4 | I |
| Cg | Cyperus glomeratus L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | +0.2 | 2.3 | − | − | − | − | − | − | 4 | I |
| Mq | Marsilea quadrifolia L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | 1.2 | − | 2.3 | 3 | I |
| Tl | Typha latifolia L. | − | − | + | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Er | Elymus repens (L.) Gould | − | + | +0.2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Vb | Veronica beccabunga L. | − | − | − | + | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Cs | Cyperus strigosus L. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 1.2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Ce | Calamagrostis epigejos (L.) Roth | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | 1.2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Pa | Phragmites australis (Cav.) Trin. ex Steud. | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 2 | I |
| Sporadic species | − | − | − | − | − | − | 1 | − | − | − | − | 1 | 1 | 1 | − | − | − | − | − | − | 3 | − | − | 1 | − | − | − | − | − | − | − | − | − | 1 | − | − | − | − | − | − | |||
| TOT | Site 1 | Sites 2–3 | Organic | Conventional | |
|---|---|---|---|---|---|
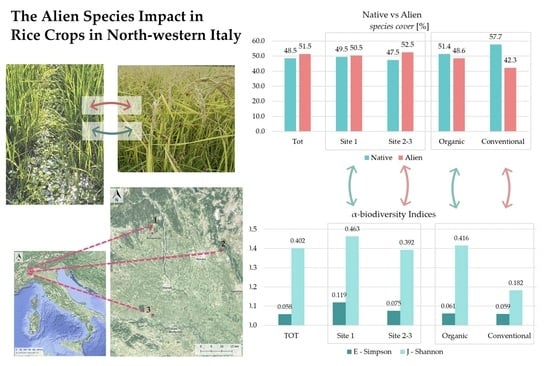
| E—Simpson | 0.058 | 0.119 | 0.075 | 0.061 | 0.059 |
| J—Shannon | 0.402 | 0.463 | 0.392 | 0.416 | 0.182 |
| Average taxa number | 8.275 | 8.933 | 7.880 | 9.135 | 5.778 |
| 1 | 2 | 3 | 4 | |
| Coverage (%) | 100 | 100 | 100 | 100 |
| Area (m2) | 90 | 80 | 80 | 80 |
| Species number | 13 | 10 | 7 | 6 |
| Oryza sativa s.l. | 3.3 | 2.3 | 1.1 | + |
| Murdannia keisak (Hassk.) Hand.−Mazz. | 4.5 | 4.4 | 5.5 | 5.5 |
| Schoenoplectiella mucronata (L.) J. Jung & H.K. Choi | 2.3 | 3.4 | 1.1 | 1.1 |
| Heteranthera reniformis Ruiz & Pav. | 3.3 | 1.2 | +0.2 | 1.2 |
| Eleocharis flavescens (Poir.) Urb. | 1.2 | 2.2 | − | − |
| Echinocloa crus−galli (L.) P. Beauv. | − | +0.2 | − | +0.2 |
| Persicaria lapathifolia (L.) Delarbre | 1.1 | 1.1 | + | − |
| Alisma plantago−aquatica L. | 1.1 | 1.2 | − | − |
| Bidens frondosa L. | 1.2 | + | + | − |
| Typha angustifolia L. | + | − | + | 1.1 |
| Typha latifolia L. | 1.1 | + | − | − |
| Polygonum aviculare L. | 1.1 | − | − | − |
| Robinia pseudoacacia L. | (+) | − | − | − |
| Salix alba L. | (+) | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagge, I.; Chiaffarelli, G. The Alien Plant Species Impact in Rice Crops in Northwestern Italy. Plants 2023, 12, 2012. https://doi.org/10.3390/plants12102012
Vagge I, Chiaffarelli G. The Alien Plant Species Impact in Rice Crops in Northwestern Italy. Plants. 2023; 12(10):2012. https://doi.org/10.3390/plants12102012
Chicago/Turabian StyleVagge, Ilda, and Gemma Chiaffarelli. 2023. "The Alien Plant Species Impact in Rice Crops in Northwestern Italy" Plants 12, no. 10: 2012. https://doi.org/10.3390/plants12102012