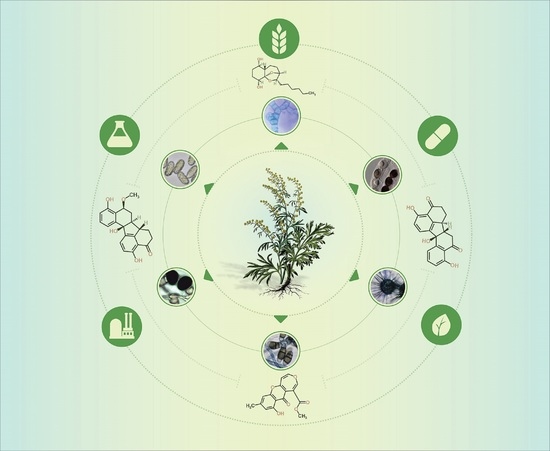
Endophytic Fungi in Species of Artemisia
Abstract
:1. State of the Art
2. Artemisia Fungal Endophyte Identification and Diversity
3. Biological Activity of Artemisia Endophytes
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Haider, F.; Dwivedi, P.D.; Naqvi, A.A.; Bagchi, G.D. Essential oil composition of Artemisia vulgaris harvested at different growth periods under indo-gangetic plain conditions. J. Essent. Oil Res. 2003, 15, 376–378. [Google Scholar] [CrossRef]
- Judžentien, A.; Buzelyte, J. Chemical composition of essential oils of Artemisia vulgaris L. (mugwort) from North Lithuania. Chemija 2006, 17, 12–15. [Google Scholar]
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of Serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, S.M. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P. The Artemisia Genus: A Review on Traditional Uses, Phytochemical Constituents, Pharmacological Properties and Germplasm Conservation. J. Glycom. Lipidom. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P. The Genus Artemisia: A 2012–2017 Literature Review on Chemical Composition, Antimicrobial, Insecticidal and Antioxidant Activities of Essential Oils. Medicines 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Youssefi, M.R.; Benelli, G. Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): Toxic and repellent potential. Parasitol. Res. 2017, 116, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zou, K.; Zhang, W.; Guo, S.; Liu, H.; Sun, J.; Li, J.; Huang, D.; Wu, Y.; Du, S.; et al. Efficacy of Compounds Isolated from the Essential Oil of Artemisia lavandulaefolia in Control of the Cigarette Beetle, Lasioderma serricorne. Molecules 2018, 23, 343. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K. Anti-inflammatory effects of eriodictyol in lipopolysaccharidestimulated raw 264.7 murine macrophages. Arch. Pharm. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Chung, H.Y.; Maier, C.G.; Wood, A.R.; Dixon, R.; Mabry, T. Estrogenic flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 1998, 46, 3325–3329. [Google Scholar] [CrossRef]
- Ikram, N.K.B.K.; Simonsen, H.T. A Review of Biotechnological Artemisinin Production in Plants. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.S.; Meurer, Y.S.R.; Oliveira, M.G.; Medeiros, W.M.T.Q.; Silva, F.O.N.; Brito, A.C.F.; Pontes, D.L.; Andrade-Neto, V. Comparative study on the antioxidant and anti-Toxoplasma activities of vanillin and its resorcinarene derivative. Molecules 2014, 19, 5898–5912. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.T.; Alshehri, M.A.; Panneerselvam, C.; Murugan, K.; Trivedi, S.; Mahyoub, J.A.; Hassan, M.M.; Maggi, F.; Sut, S.; Dall’Aqua, S.; et al. The desert wormwood (Artemisia herba-alba)—From Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. J. Photochem. Photobiol. B Biol. 2018, 180, 225–234. [Google Scholar] [CrossRef] [PubMed]
- D’Addabbo, T.; Argentieri, M.P.; Radicci, V.; Grassi, F.; Avato, P. Artemisia annua compounds have potential to manage root-knot and potato cyst nematodes. Ind. Crops Prod. 2017, 108, 195–200. [Google Scholar] [CrossRef]
- Julio, L.F.; González-Coloma, A.; Burillo, J.; Diaz, C.E.; Andrés, M.F. Nematicidal activity of the hydrolate byproduct from the semi industrial vapor pressure extraction of domesticated Artemisia absinthium against Meloidogyne javanica. Crop Prot. 2017, 94, 33–37. [Google Scholar] [CrossRef]
- Chengala, L. Botanical pesticides—A major alternative to chemical pesticides: A review. Int. J. Life Sci. 2017, 5, 722–729. [Google Scholar]
- Esmaeily, M.; Bandani, A.; Zibaee, I.; Sharifian, I.; Zare, S. Sublethal effects of Artemisia annua L. and Rosmarinus officinalis L. essential oils on life table parameters of Tetranychus urticae (Acari: Tetranychidae). Persian J. Acarol. 2017, 6, 39–52. [Google Scholar]
- Sainz, P.; Cruz-Estrada, Á.; Díaz, C.E.; González-Coloma, A. The genus Artemisia: Distribution and phytochemistry in the Iberian Peninsula and the Canary and Balearic Islands. Phytochem. Rev. 2017, 16, 1023–1043. [Google Scholar] [CrossRef]
- Royal Botanic Gardens K and MBG. The Plant List 2010. Available online: http://www.theplantlist.org/tpl/search?q=artemisia (accessed on 3 February 2017).
- Huang, W.Y.; Cai, Y.Z.; Surveswaran, S.; Hyde, K.D.; Corke, H.; Sun, M. Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers. 2009, 36, 69–88. [Google Scholar]
- Cosoveanu, A.; Hernandez, M.; Iacomi-Vasilescu, B.; Zhang, X.; Shu, S.; Wang, M.; Cabrera, R. Fungi as endophytes in Chinese Artemisia spp.: Juxtaposed elements of phylogeny, diversity and bioactivity. Mycosphere 2016, 7, 102–117. [Google Scholar] [CrossRef]
- Cosoveanu, A.; Rodriguez Sabina, S.; Cabrera, R. Fungi as endophytes in Artemisia thuscula: Juxtaposed elements of diversity and phylogeny. J. Fungi 2018, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.L.; Chen, Y.C.; Ma, X.J. Symbiotic fungi in roots of Artemisia annua with special reference to endophytic colonizers. Plant Biosyst. 2011, 145, 495–502. [Google Scholar] [CrossRef]
- Ofek-lalzar, M.; Gur, Y.; Ben-Moshe, S.; Sharon, O.; Kosman, E.; Mochli, E.; Sharon, A. Diversity of fungal endophytes in recent and ancient wheat ancestors of Triticum dicoccoides and Aegilops sharonensis. FEMS Microbiol. Ecol. Adv. 2016, 92, 1–26. [Google Scholar] [CrossRef]
- Kernaghan, G.; Mayerhofer, M.; Griffin, A. Fungal endophytes of wild and hybrid Vitis leaves and their potential for vineyard biocontrol. Can. J. Microbiol. 2017, 63, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.M.C.; Hyde, K.D.; Corlett, R.T. Diversity of fungi on wild fruits in Hong Kong. Fungal Divers. 2003, 14, 165–185. [Google Scholar]
- Qian, Y.; Kang, J.; Geng, K.; Wang, L.; Lei, B. Endophytic fungi from Artemisia argyi Levl. et Vant. and their bioactivity. Chiang Mai J. Sci. 2014, 41, 910–921. [Google Scholar]
- Myrchiang, P.; Dkhar, M.S.; Devi, H.R. Studies on endophytic fungi associated with medicinally important aromatic plant Artemisia nilagirica (C.B. Clarke) Pamp. and their antagonistic activity against Phytophthora infestans. J. Adv. Lab. Res. Biol. 2014, 5, 112–119. [Google Scholar]
- Cosoveanu, A.; Da Silva, E.; Gimenez Marino, C.; Nunez Trujillo, G.; Gonzalez Coloma, A.; Frias Viera, I.; Cabrera, R. Artemisia thuscula Cav.: Antibacterial, antifungal activity of the plant extracts and associated endophytes. J. Hortic. For. Biotechnol. 2012, 16, 87–90. [Google Scholar]
- Gautam, A.K. Diversity of fungal endophytes in some medicinal plants of Himachal Pradesh, India. Arch. Phytopathol. Plant Prot. 2014, 47, 537–544. [Google Scholar] [CrossRef]
- Chowdhary, K.; Kaushik, N. Fungal endophyte diversity and bioactivity in the Indian medicinal plant Ocimum sanctum Linn. PLoS ONE 2015, 10, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhou, Y.; Zhou, X.; Xia, X.; Wei, Y.; He, L.; Tang, H.; Yu, L. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 5929. [Google Scholar] [CrossRef] [PubMed]
- Nalini, M.S.; Sunayana, N.; Prakash, H.S. Endophytic Fungal Diversity in Medicinal Plants of Western Ghats, India. Int. J. Biodivers. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Sánchez Márquez, S.; Bills, G.F.; Herrero, N.; Zabalgogeazcoa, Í. Non-systemic fungal endophytes of grasses. Fungal Ecol. 2012, 5, 289–297. [Google Scholar] [CrossRef]
- Banerjee, D. Endophytic fungal diversity in tropical and subtropical plants. Res. J. Microbiol. 2011, 6, 54–62. [Google Scholar] [CrossRef]
- Casieri, L.; Hofstetter, V.; Viret, O.; Gindro, K. Fungal communities in the wood of different cultivars of young Vitis vinifera plants. Phytopathol. Mediterr. 2009, 48, 73–83. [Google Scholar] [CrossRef]
- Qian, Y.; Kang, J.; Lei, B.; Wang, L.; Huang, Y. Screening and taxonomic identification of endophytic fungi with antitumor and antioxidant activities from Artemisia lactiflora. China J. Chin. Mater. Med. 2014, 39, 438–441. [Google Scholar]
- Lu, H.; Zou, W.X.; Meng, J.C.; Hu, J.; Tan, R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000, 151, 67–73. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, X.; Wu, B. Evaluation of antimicrobial activities of extracts of endophytic fungi from Artemisia annua. Bangladesh J. Pharmacol. 2012, 7, 120–123. [Google Scholar] [CrossRef]
- Gu, W.; Ge, H.M.; Song, Y.C.; Ding, H.; Zhu, H.L.; Zhao, X.A.; Tan, R.X. Cytotoxic benzo[j]fluoranthene metabolites from Hypoxylon truncatum IFB-18, an endophyte of Artemisia annua. J. Nat. Prod. 2007, 70, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Liu, C.H.; Zou, W.X.; Tan, R.X. Leptosphaerone, a metabolite with a novel skeleton from Leptosphaeria sp. IV403, an endophytic fungus in Artemisia annua. Helv. Chim. Acta 2002, 85, 2662–2667. [Google Scholar] [CrossRef]
- Wilco, E.P.; Verberk, E.P. Explaining general patterns in species abundance and distributions. Nat. Educ. Knowl. 2011, 3, 38. [Google Scholar]
- Galand, P.E.; Casamayor, E.O.; Kirchman, D.L.; Lovejoy, C. Ecology of the rare microbial biosphere of the Arctic Ocean. Proc. Natl. Acad. Sci. USA 2009, 106, 22427–22432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, A.E.; Mejia, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed]
- Dastogeer, K.M.G.; Li, H.; Sivasithamparam, K.; Jones, M.G.K.; Wylie, S.J. Host Specificity of Endophytic Mycobiota of Wild Nicotiana Plants from Arid Regions of Northern Australia. Microb. Ecol. 2018, 75, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ding, Q.; Hyde, K.D.; Guo, L.D. Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecol. 2012, 5, 624–632. [Google Scholar] [CrossRef]
- Zhou, D.; Hyde, K.D. Host specificity, hos exclusivity and host-recurrence in saprobic fungi. Mycol. Res. 2001, 105, 1449–1457. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Li, S.; Shi, D.H.; Song, Y.C.; Tan, R.X. Chemical constituents of liquid culture of endophyte IFB-E012 in Artemisia annua. Chin. J. Nat. Med. 2009, 7, 354–356. [Google Scholar] [CrossRef]
- Liu, C.H.; Zou, W.X.; Lu, H.; Tan, R.X. Antifungal activity of Artemisia annua endophyte cultures against phytopathogenic fungi. J. Biotechnol. 2001, 88, 277–282. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhang, Z.; Tan, R.X. Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colletotrichum sp. Biotechnol. Lett. 2001, 23, 857–860. [Google Scholar] [CrossRef]
- Wang, J.W.; Zheng, L.P.; Tan, R.X. The preparation of an elicitor from a fungal endophyte to enhance artemisinin production in hairy root culture of Artemisia annua L. Chin. J. Biotechnol. 2006, 22, 829–834. [Google Scholar]
- Liu, J.Y.; Liu, C.H.; Zou, W.X.; Tan, R.X. Leptosphaeric acid, a metabolite with a novel carbon skeleton from Leptosphaeria sp. IV403, an endophytic fungus in Artemisia annua. Helv. Chim. Acta 2003, 86, 657–660. [Google Scholar] [CrossRef]
- Arora, M.; Saxena, P.; Choudhary, D.K.; Abdin, M.Z.; Varma, A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016, 32, 19. [Google Scholar] [CrossRef] [PubMed]
- Baishya, D.; Deka, P.; Kalita, M.C. In vitro co-cultivation of Piriformospora indica filtrate for improve biomass productivity in Artemisia annua (L.). Symbiosis 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Jogawat, A.; Saha, S.; Bakshi, M.; Dayaman, V.; Kumar, M.; Dua, M.; Varma, M.; Oelmuller, R.; Tuteja, N.; Johri, A.K. Piriformospora indica rescues growth diminution of rice seedlings during high salt stress. Plant Signal Behav. 2013, 8, e26891. [Google Scholar] [CrossRef]
- Hussain, M.; Mahajan, V.; Rather, I.A.; Awasthi, P.; Chouhan, R.; Dutt, P.; Sharma, Y.P.; Bedi, Y.S.; Gandhi, S.G. Isolation and identification of growth promoting endophytic fungi from Artemisia annua L. and its effects on artemisinin content. Trends Phytochem. Res. 2017, 1, 207–214. [Google Scholar]
- Jessing, K.K.; Duke, S.O.; Cedergreeen, N. Potential Ecological Roles of Artemisinin Produced by Artemisia annua L. J. Chem. Ecol. 2014, 40, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K. Anticancer effect of Antimalarial artemisinin compounds. Ann. Med. Health Sci. Res. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Xia, Z.H.; Tan, R.X. Elicitation on Artemisinin Biosynthesis in Artemisia annua Hairy Roots by the Oligosaccharide extract from the Endophytic Colletotrichum sp. B501. Acta Bot. Sin. 2002, 44, 1233–1238. [Google Scholar]
- Zheng, L.P.; Tian, H.; Yuan, Y.F.; Wang, J.W. The influence of endophytic Penicillium oxalicum B4 on growth and artemisinin biosynthesis of in vitro propagated plantlets of Artemisia annua L. Plant Growth Regul. 2016, 80, 93–102. [Google Scholar] [CrossRef]
- Zou, W.X.; Meng, J.C.; Lu, H.; Chen, G.X.; Shi, G.X.; Zhang, T.Y.; Tan, R.X. Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. J. Nat. Prod. 2000, 63, 1529–1530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Ying, C.; Tang, Y.F. Four ardeemin analogs from endophytic Aspergillus fumigatus SPS-02 and their reversal effects on multidrug-resistant tumor cells. Chem. Biodivers. 2014, 11, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Purwantini, I.; Wahyono, M.; Ratna, A. Isolation of endophytic fungi from Artemisia annua, L and identification of their antimicrobial compound using bioautography method. Int. J. Phrmacy Pharm. Sci. 2015, 7, 95–99. [Google Scholar]
- Hsieh, P.W.; Hsu, L.C.; Lai, C.H.; Wu, C.C.; Hwang, T.L.; Lin, Y.K.; Wu, Y.C. Evaluation of the bioactivities of extracts of endophytes isolated from Taiwanese herbal plants. World J. Microbiol. Biotechnol. 2009, 25, 1461–1469. [Google Scholar] [CrossRef]
- Shi, X.S.; Wang, D.J.; Li, X.M.; Li, H.L.; Meng, L.H.; Li, X.; Pi, Y.; Zhou, X.W.; Wang, B.G. Antimicrobial polyketides from Trichoderma koningiopsis QA-3, an endophytic fungus obtained from the medicinal plant Artemisia argyi. RSC Adv. 2017, 7, 51335–51342. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, L.; Tan, Q.; Wan, D.; Xie, J.; Peng, J. New cytotoxic trichothecene macrolide epimers from endophytic Myrothecium roridum IFB-E012. J. Antibiot. 2016, 69, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Jiao, R.H.; Ye, Y.H.; Wang, X.T.; Xu, C.; Song, Y.C.; Zhu, H.L.; Tan, R.X. Absolute configuration of new cytotoxic and other bioactive trichothecene macrolides. Chem. A Eur. J. 2006, 12, 5596–5602. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.N.; Hashimoto, T.; Nomura, Y.; Wollweber, H.; Hellwig, V.; Fournier, J.; Staedler, M.; Asakawa, Y. Cohaerins A and B, azaphilones from the fungus Hypoxylon cohaerens, and comparison of HPLC-based metabolite profiles in Hypoxylon sect. Annulata. Phytochemistry 2005, 66, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cai, Y.Z.; Xing, J.; Corke, H.; Sun, M. A potential antioxidant resource: Endophytic fungi from medicinal plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Noumeur, S.R.; Mancini, V.; Romanazzi, G. Activity of endophytic fungi from Artemisia absinthium on Botrytis cinerea. Acta Hortic. 2016, 1144, 101–104. [Google Scholar] [CrossRef]
- Tian, H.; Ma, Y.J.; Li, W.Y.; Wang, J.W. Efficient degradation of triclosan by an endophytic fungus Penicillium oxalicum B4. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Zhang, J.; Hu, S.; Zhang, Z.J.; Zhu, C.J.; Ng, S.W.; Tan, R.W. Ardeemins and cytochalasins from Aspergillus terreus residing in Artemisia annua. Planta Med. 2010, 76, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, J.S.; Shen, H.J.; Song, Y.C.; Tan, R.X. A new cytotoxic trichothecene macrolide from the endophyte Myrothecium roridum. Planta Med. 2010, 76, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.M.; Song, Y.C.; Chen, J.R.; Hu, S.; Wu, J.Y.; Tan, R.X. Paranolin: A new xanthene-based metabolite from Paraphaeosphaeria nolinae. Helv. Chim. Acta 2006, 89, 502–506. [Google Scholar] [CrossRef]
- Ngankaranatikarn, P.; Chuanasa, T.; Sriubolmas, N.; Suwanborirux, K. Antileukemic activity and secondary metabolites of an endophytics fungus Phomopsis sp. from Artemisia annua. Thai J. Pharm. Sci. 2013, 38, 195–198. [Google Scholar]
- Lösgen, S.; Magull, J.; Schulz, B.; Draeger, S.; Zeeck, A. Isofusidienols: Novel chromone-3-oxepines produced by the endophytic fungus Chalara sp. Eur. J. Org. Chem. 2008, 698–703. [Google Scholar] [CrossRef]


| Order RA % | Family RA % | Genus | Genus RA % Per Host Plant/Plants | Host Plants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphisphaeriales 1.92 | Pestalotiopsidaceae 2.13 | Pestalotiopsis | 0.9 | 1.19 | 11.76 | A. capillaris* + A. indica* + A. lactiflora* (HK); A. thuscula (TF); A. argy* (CH) | ||||||||
| Botryosphaeriales 7.69 | Aplosporellaceae 2.13 | Aplosporella | 1.22 | A. thuscula (TF) | ||||||||||
| Botryosphaeriaceae 6.38 | Botryosphaeria | 41.67 | A. lavandulifolia (CH) | |||||||||||
| Macrophomina | 2.44 | A. thuscula (TF) | ||||||||||||
| Neofusicoccum | 2.78 | 30.3 | 10.98 | A. lavandulifolia; A. thuscula (LP); A. thuscula (TF) | ||||||||||
| Cantharellales 1.92 | Ceratobasidiaceae 2.13 | Rhizoctonia | 16.67 | A. brachyloba (CH) | ||||||||||
| Capnodiales 3.85 | Cladosporiaceae 2.13 | Cladosporium | 2.44 | 2.77 | 5.88 | 8.69 | A. thuscula (TF); A. annua* (CH); A. argy*; A. nilagirica* (IN) | |||||||
| Dissoconiaceae 2.13 | Ramichloridium | 2.77 | A. annua* | |||||||||||
| Diaporthales 3.85 | Diaporthaceae 4.26 | Diaporthe | 5.56 | 16.67 | 8.33 | 9.09 | 5.88 | A. lavandulifolia; A. brachyloba; A. scoparia (CH); A. thuscula (LP); A. argy* | ||||||
| Phomopsis | 6.3 | 5.88 | 36.36 | A. capillaris +A. indica + A. lactiflora*; A. argy*; A. lactiflora* (CH) | ||||||||||
| Dothideales 1.92 | Dothioraceae 2.13 | Aureobasidium | 0.9 | 3.66 | A. capillaris + A. indica + A. lactiflora*; A. thuscula (TF) | |||||||||
| Eurotiales 7.69 | Trichocomaceae 8.51 | Aspergillus | 8.33 | 4.55 | 8.69 | A. lavandulifolia; A. thuscula (LP); A. nilagirica* | ||||||||
| Eupenicillium | 5.55 | A. annua* | ||||||||||||
| Paecilomyces | 2.77 | A. annua* | ||||||||||||
| Penicillium | 2.78 | 1.52 | 13.8 | 5.88 | 33.33 | 13.4 | A. lavandulifolia; A. thuscula (LP); A. annua*; A. argy*; A. nilagirica*; A. annua | |||||||
| Glomerellales 1.92 | Glomerellaceae 2.13 | Colletotrichum | 19.81 | 11.1 | 25 | 5.55 | 5.88 | 10.11 | 9.09 | A. capillaris + A. indica + A. lactiflora*; A. lavandulifolia; A. brachyloba; A. annua*; A. argy*; A. annua; A. lactiflora* (CH) | ||||
| Hypocreales 17.31 | Clavicipitaceae 2.13 | Ephelis | 1.8 | A. capillaris + A. indica + A. lactiflora* | ||||||||||
| Hypocreaceae 4.26 | Hypocrea | 5.88 | 9.09 | A. argy*; A. lactiflora* | ||||||||||
| Trichoderma | 4.34 | A. nilagirica* | ||||||||||||
| Nectriaceae 4.26 | Fusarium | 5.55 | 5.88 | 4.34 | A. annua*; A. argy*; A. nilagirica* | |||||||||
| Nectria | 2.44 | A. thuscula (TF) | ||||||||||||
| Stachybotryaceae 4.26 | Stachybotrys | 1.22 | A. thuscula (TF) | |||||||||||
| Myrothecium | N.A. | A. nilagirica* | ||||||||||||
| Acremonium | 13 | A. annua | ||||||||||||
| Cephalosporium | N.A. | A. annua | ||||||||||||
| Mucorales 3.85 | Mucoraceae4.26 | Rhizopus | 4.34 | A. nilagirica* | ||||||||||
| Mucor | N.A. | A. annua | ||||||||||||
| Onygenales 1.92 | Arthrodermataceae 2.13 | Arthroderma | 4.34 | A. nilagirica* | ||||||||||
| Pleosporales 25.00 | Sporormiaceae 2.13 | Preussia | 10.61 | 10.98 | A. thuscula (LP); A. thuscula (TF) | |||||||||
| Didymosphaeriaceae 2.13 | Tremateia | 1.52 | A. thuscula (LP) | |||||||||||
| Leptosphaeriaceae 4.26 | Coniothyrium | 1.52 | A. thuscula (LP) | |||||||||||
| Leptosphaeria | N.A. | A. annua | ||||||||||||
| Pleosporaceae 14.89 | Alternaria | 24.30 | 13.89 | 75 | 25 | 66.67 | 33.33 | 33.33 | 23.5 | 36.36 | 28.79 | 41.46 | A. capillaris + A. indica + A. lactiflora*; A. lavandulifolia; A. tangutica; A. brachyloba; A. subulata; A. argy; A. scoparia; A. argy*; A. lactiflora*; A. thuscula (LP); A. thuscula (TF) | |
| Curvularia | 16.66 | 33.33 | 16.67 | 8.33 | 1.52 | A. tangutica (CH); A. brachyloba (CH); A. subulata (CH); A. argy; A. thuscula (LP) | ||||||||
| Drechslera | 0.9 | A. capillaris + A. indica + A. lactiflora* | ||||||||||||
| Edenia | 2.77 | A. annua* | ||||||||||||
| Neoplatysporoides | 3.03 | A. thuscula (LP) | ||||||||||||
| Stemphylium | 0.65 | 4.88 | A. thuscula (LP); A. thuscula (TF) | |||||||||||
| Paraphoma | 1.52 | A. thuscula (LP) | ||||||||||||
| Phoma | 1.52 | 7.32 | 2.77 | 4.34 | A. thuscula (LP); A. thuscula (TF); A. annua*; A. nilagirica* | |||||||||
| Pleosporineae 1.92 | Camarosporiaceae 2.13 | Camarosporium | 1.52 | 1.22 | A. thuscula (LP); A. thuscula (TF) | |||||||||
| Pythiales 1.92 | Pythiaceae 2.13 | Pythium | 4.34 | A. nilagirica* | ||||||||||
| Saccharomycetales 1.92 | Debaryomycetaceae 2.13 | Meyerozyma | 9.09 | A. lactiflora* | ||||||||||
| Sordariales 3.85 | Chaetomiaceae 4.26 | Chaetomium | 2.44 | 11.76 | A. thuscula (TF); A. argy* | |||||||||
| Thielavia | 2.44 | A. thuscula (TF) | ||||||||||||
| Sporidiobolales 1.92 | Sporidiobolaceae 2.13 | Rhodotorula | 5.88 | A. argy* | ||||||||||
| Xylariales 9.62 | Apiosporaceae 4.26 | Arthrinium | 5.88 | A. argy* | ||||||||||
| Nigrospora | 5.56 | 33.33 | 75 | 25 | 3.03 | A. lavandulifolia; A. brachyloba; A. argy; A. scoparia; A. thuscula (LP) | ||||||||
| Graphostromataceae 2.13 | Biscogniauxia | 2.44 | A. thuscula (TF) | |||||||||||
| Hypoxylaceae 2.13 | Hypoxylon | N.A. | A. annua | |||||||||||
| Artemisia Species | Plant Part | EF Taxa | EF Identification Method | Compound/Extract | Class of Compounds | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| A. absinthium | root | Penicillium spp. (strains B6C18 and B14C36) | N.A. | spore solution (106 mL−1) | N.A. | Antifungal inhibition of diameter of lesions on vine berries: 75% and 91% | [73] |
| A. annua | stem | Aspergillus spp. | morphology | ethyl acetate crude extract | N.A. | Antibacterial, antifungal | [41] |
| Mucor spp. | |||||||
| Cephalosporium spp. | |||||||
| Fusarium spp. | Antibacterial | ||||||
| Aspergillus terreus (strain IFB-E030) | N.A. | 10-phenyl-[12]-cytochalasins Z17 | Cytochalasan, alkaloids | Cytotoxic IC50 = 26.2 µM | [75] | ||
| Colletotrichum sp. | morphology | 3β-hydroxy-ergosta-5-ene | Ergosterol derivatives | Antifungal %I (200 µg mL−1): 77–85% | [40] | ||
| Antimicrobial MIC (µg mL−1): 50–75 | |||||||
| 3-oxo-ergosta-4,6,8(11),22-tetraene | Triunsaturated steroids | Antifungal %I (200 µg mL−1): 75% | |||||
| Antimicrobial MIC (µg mL−1): 25–75% | |||||||
| 3β-hydroxy-5α,8α-epidioxy-ergosta-6,22-diene | Ergosterol peroxides | Antimicrobial MIC (µg mL−1): 50–75 | |||||
| 6-isoprenylindole-3-carboxylic acid | indoles | Antifungal %I (200 µg mL−1): 57–77% | |||||
| Antimicrobial MIC (µg mL−1): 25–75 | |||||||
| 3β,5α-dihydroxy-6β-acetoxy-ergosta-7,22-diene | sterols | Antifungal %I (200 µg mL−1): 75–77% | |||||
| Antimicrobial MIC (µg mL−1): 50–100% | |||||||
| 3β,5α-dihydroxy-6β-phenylacety- loxy-ergosta-7,22-diene | Antifungal %I (200 µg mL−1): 50–66% | ||||||
| Antimicrobial MIC (µg mL−1): 50–75 | |||||||
| Hypoxylon trunctatum | morphology and 18S rDNA sequence | daldinone C | Benzo[j]fluoranthenes (polycyclic aromatic hydrocarbons) | Cytotoxic IC50 = 49.5 µM | [42] | ||
| daldinone D | Cytotoxic IC50 = 41 µM | ||||||
| Myrothecium roridum (strain IFB-E012) | myrothecine A | Sesquiterpene-based trichothecenes | Cytotoxic IC50 = 8.5 µg mL−1 | [69,76] | |||
| myrothecine B | Cytotoxic IC50 = 0.76 µg mL−1 | ||||||
| myrothecine C | Cytotoxic IC50 = 32.21 µg mL−1 | ||||||
| roridin E | Macrocyclic trychothecenes | Cytotoxic IC50 = 0.03 µg mL−1 | |||||
| mytoxin B | Trychothecene macrolides | Cytotoxic IC50 = 0.002 µg mL−1 | |||||
| dihydromyrothecine C | Cytotoxic IC50 = 44.48 µM | ||||||
| roritoxin E | Cytotoxic IC50 = 0.26 µg mL−1, 10.54 µg mL−1 | ||||||
| Aspergillus fumigatus | 5-N-acetylardeemin | Pyrimidinones (aromatic heterocyclic diazenes) | Cytotoxic RF = 1.5–6 µM | [65] | |||
| 5-N-acetyl-15bβ-hydroxyardeemin | Cytotoxic RF = 2–9.5 µM | ||||||
| 5-N-acetyl-15b-didehydroardeemin | Cytotoxic RF = 2.5–8 µM | ||||||
| 5-N-acetyl-16α-hydroxyardeemin | Cytotoxic RF = 1.5–7 µM | ||||||
| Colletotrichum gloeosporioides | morphology | oligosaccharides extract elicitor for artemisinin | oligosaccharides | increment of artemisinin = 64.29% | [62] | ||
| Penicillium oxalicum | ITS1, 5.8S, and ITS2 | heat-killed mycelium degrading triclosan (TCS) | N.A. | max. adsorbing capacity of TCS = 17.60 mg g−1 | [74] | ||
| leaves | Curvularia pallescens | elicitor extract for artemisinin | N.A. | 2–6% elicitor extract (w/v): artemisinin content = 1.21–3.47-times more than control | [59] | ||
| Cladosporium sp. | morphology | ethyl acetate extract | N.A. | Antibacterial | [66] | ||
| stem | Paraphaeosphaeria nolinae (strain IFB-E011) | N.A. | paranolin | polycyclic aromatic compounds (xanthene-based) | Cytotoxic IC50 values > 50 µg mL−1 | [77] | |
| Phomopsis sp. | crude EtOAc extract = BB1, fraction Hex/EtOAc/MeOH = BB4, fractions MeOH = BB8, BB9, BB10 | N.A. | Cytotoxic: IC50 (µg mL−1) BB1 = 17.11, BB4 = 0.7; %I (20 µg/mL) BB8 = 1.97, BB9 = 0.53, BB10 = 52.98 | [78] | |||
| tyrosol (purified from BB10) | Phenolic compounds | ||||||
| A. argyi | N.A. | Phomopsis sp. (strain H31) | ITS1, 5.8S, and ITS2 | ethyl acetate + ethyl acetate/water extract | N.A. | Antifungal %I (1 mg mL−1) = 34.57; Cytotoxic %I (20 µg mL−1) = 81.69 | [29] |
| Cladosporium sp. (strain H23) | Antifungal %I (1 mg mL−1) = 23.46 | ||||||
| Chaetomium sp. (strain H40) | Antibacterial | ||||||
| Penicillium sp. (strain H9) | Antibacterial | ||||||
| Pestalotiopsis sp. (strain H14) | Antibacterial; Cytotoxic %I (20 µg mL−1) = 53.47, 71.43, 97.03 | ||||||
| Diaporthe sp. (strain H26) | Antibacterial | ||||||
| Trichoderma sp. (strain H17) | Antibacterial | ||||||
| Rhodotorula sp. (strain H13) | Cytotoxic %I (20 µg mL−1) = 16.41, 23.56, 78.28 | ||||||
| Colletotrichum sp. (strain H42) | Cytotoxic %I (20 µg mL−1) = 11.22, 26.95, 71.99 | ||||||
| stem | Nigrospora sphaerica | morphology and ITS1, 5.8S, and ITS2 | ethyl acetate crude extract | N.A. | Antifungal %I (1 mg mL−1): 48.37, 11.94, 24.92 | [23] | |
| Trichoderma koningiopsis (strain QA-3) | ITS1, 5.8S, and ITS2 | ent-koninginin A | Tricyclic polyketides | Antifungal MIC (µg mL−1): 8–64 Antibacterial MIC (µg mL−1): 8–64 | [68] | ||
| 1,6-di-epi-koninginin A | Antifungal MIC (µg mL−1): 64 Antibacterial MIC (µg mL−1): 32–64 | ||||||
| 15-hydroxykoninginin A | Antifungal MIC (µg mL−1): 64 Antibacterial MIC (µg mL−1): 16–64 | ||||||
| 10-deacetylkoningiopisin | Bicyclic polyketides | Antifungal MIC (µg mL−1): 8–64 Antibacterial MIC (µg mL−1): 32–64 | |||||
| koninginin T | Tricyclic polyketides | Antifungal MIC (µg mL−1): 16 Antibacterial MIC (µg mL−1): 32–64 | |||||
| koninginin L | Antifungal MIC (µg mL−1): 16 Antibacterial MIC (µg mL−1): 32–64 | ||||||
| trichoketide A | Bicyclic polyketides | Antifungal MIC (µg mL−1): 4–64 Antibacterial MIC (µg mL−1): 16–64 | |||||
| A. brachyloba | Alternaria alternata | morphology and ITS1, 5.8S, and ITS2 | ethyl acetate crude extract | N.A. | Antifungal %I (1 mg mL−1): 29.8, 33.2, 42.4 | [23] | |
| A. capillaris | flower | Alternaria sp. (strain Acap F1) | ITS1, 5.8S, and ITS2 | ethyl acetate extract of mycelia; Trolox equivalent antioxidant content (TEAC), Total phenolic content (TPC) | phenolic acids, flavonoids | TEAC (µM trolox equivalent/100 mL culture) = 127.41; TPC (mg gallic acid equivalent/100 mL culture) = 13.71 | [22,72] |
| Phomopsis sp. (strain Acap. F3) | TEAC (µM trolox equivalent/100 mL culture) = 526.93; TPC (mg gallic acid equivalent/100 mL culture) = 49.22 | ||||||
| Phomopsis sp. (strain AcapF4) | TEAC (µM trolox equivalent/100 mL culture) = 177.12; TPC (mg gallic acid equivalent/100 mL culture) = 18.43 | ||||||
| A. indica | leaves | Colletotrichum sp. (strain AiL1) | TEAC (µM trolox equivalent/100 mL culture) = 100.2; TPC (mg gallic acid equivalent/100 mL culture) = 6.88 | ||||
| Xylaria sp. (strain AiL3) | TEAC (µM trolox equivalent/100 mL culture) = 102.21; TPC (mg gallic acid equivalent/100 mL culture) = 8.69 | ||||||
| Diaporthe sp. (strain AiL4) | TEAC (µM trolox equivalent/100 mL culture) = 217.76; TPC (mg gallic acid equivalent/100 mL culture) = 21.27 | ||||||
| A. lactiflora | N.A. | Phomopsis sp. (strain GYBH42) | ethyl acetate crude extract | N.A. | Cytotoxic, antioxidant | [39] | |
| Alternaria sp. (strain GYBH47) | |||||||
| A. lavandulifolia | stem | Cochliobolus geniculatus | morphology and ITS1, 5.8S, and ITS2 | Antifungal EC50 = 0.03; %I (1 mg mL−1) = 31.5 and 100 | [23] | ||
| Botryosphaeria dothidea | Antifungal %I (1 mg mL−1): 85.79, 22.4 | ||||||
| A. mongolica | Colletotrichum gloeosporioides | morphology | colletotric acid | Phenolic acids | Antibacterial MIC (µg mL−1): 25–50 | [64] | |
| A. subulata | Curvularia spicifera | morphology and ITS1, 5.8S, and ITS2 | ethyl acetate crude extract | N.A. | Antifungal %I (1 mg mL−1): 8.95, 52.09, 77.9 | [23] | |
| A. vulgaris | N.A. | Chalara sp. (strain 6661) | N.A. | isofusidienol A, B, C, and D | chromones (benzopyran derivatives) | Antibacterial | [79] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosoveanu, A.; Cabrera, R. Endophytic Fungi in Species of Artemisia. J. Fungi 2018, 4, 53. https://doi.org/10.3390/jof4020053
Cosoveanu A, Cabrera R. Endophytic Fungi in Species of Artemisia. Journal of Fungi. 2018; 4(2):53. https://doi.org/10.3390/jof4020053
Chicago/Turabian StyleCosoveanu, Andreea, and Raimundo Cabrera. 2018. "Endophytic Fungi in Species of Artemisia" Journal of Fungi 4, no. 2: 53. https://doi.org/10.3390/jof4020053