Inter-Otolith Differences in Strontium Markings: A Case Study on the Juvenile Crucian Carp Carassius carassius (Linnaeus, 1758)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Extraction and Detection of Otoliths
2.3. Data Processing
3. Results
3.1. Effect of Sr Marking on the Survival and Growth of Juvenile Crucian Carp
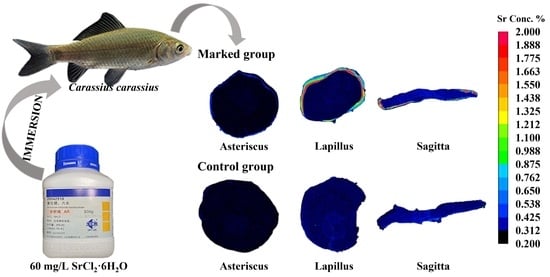
3.2. Otolith Marking
3.2.1. Inter-Otolith Variation in Sr/Ca Ratio
3.2.2. Inter-Otolith Changing of Sr Map Patterns
3.2.3. Inter-Otolith Time Delay for Sr Marking
4. Discussion
4.1. Selection of Otolith Type for Observation of Sr Marking
4.2. Variations in Sr-Marked Effects among the Three Types of Otoliths
4.3. Significance of Time Delay to Sr Markings of Otoliths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren-Myers, F.; Dempster, T.; Swearer, S.E. Otolith mass marking techniques for aquaculture and restocking: Benefits and limitations. Rev. Fish. Biol. Fish. 2018, 28, 485–501. [Google Scholar] [CrossRef]
- Morita, K.; Saito, T.; Miyakoshi, Y.; Fukuwaka, M.-A.; Nagasawa, T.; Kaeriyama, M. A review of Pacific salmon hatchery programmes on Hokkaido Island, Japan. ICES J. Mar. Sci. 2006, 63, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Avigliano, E.; Martinez, C.F.R.; Volpedo, A.V. Combined use of otolith microchemistry and morphometry as indicators of the habitat of the silverside (Odontesthes bonariensis) in a freshwater–estuarine environment. Fish. Res. 2014, 149, 55–60. [Google Scholar] [CrossRef]
- Fowler, A.M.; Smith, S.M.; Booth, D.J.; Stewart, J. Partial migration of grey mullet (Mugil cephalus) on Australia’s east coast revealed by otolith chemistry. Mar. Environ. Res. 2016, 119, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.D.; Kozdon, R.; Blum, M.J.; Gilliam, J.F.; Valley, J.W.; Mcintyre, P.B. Reconstructing larval growth and habitat use in an amphidromous goby using otolith increments and microchemistry. J. Fish Biol. 2017, 90, 1338–1355. [Google Scholar] [CrossRef] [PubMed]
- Taddese, F.; Reid, M.R.; Closs, G.P. Direct relationship between water and otolith chemistry in juvenile estuarine triplefin Forsterygion nigripenne. Fish. Res. 2019, 211, 32–39. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef] [Green Version]
- Elsdon, T.S.; Gillanders, B.M. Fish otolith chemistry influenced by exposure to multiple environmental variables. J. Exp. Mar. Biol. Ecol. 2004, 313, 269–284. [Google Scholar] [CrossRef]
- Bath Martin, G.; Thorrold, S.R. Temperature and salinity effects on magnesium, manganese, and barium incorporation in otoliths of larval and early juvenile spot Leiostomus xanthurus. Mar. Ecol.-Prog. Ser. 2005, 293, 223–232. [Google Scholar] [CrossRef]
- Izzo, C.; Reis-Santos, P.; Gillanders, B.M. Otolith chemistry does not just reflect environmental conditions: A meta-analytic evaluation. Fish. Fish. 2018, 19, 441–454. [Google Scholar] [CrossRef]
- Davoren, G.K.; Halden, N.M. Connectivity of capelin (Mallotus villosus) between regions and spawning habitats in Newfoundland inferred from otolith chemistry. Fish. Res. 2014, 159, 95–104. [Google Scholar] [CrossRef]
- Crook, D.A.; Gillanders, B.M. Use of otolith chemical signatures to estimate carp recruitment sources in the mid-Murray River, Australia. River Res. Appl. 2006, 22, 871–879. [Google Scholar] [CrossRef]
- Avigliano, E.; Carvalho, B.M.D.; Leisen, M.; Romero, R.; Velasco, G.; Vianna, M.; Barra, F.; Volpedo, A.V. Otolith edge fingerprints as approach for stock identification of Genidens barbus. Estuar. Coast Shelf Sci. 2017, 194, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, and elements: Keys to a comprehensive understanding of fish populations? Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Hayden, T.A.; Limburg, K.E.; Pine, W.E. Using otolith chemistry tags and growth patterns to distinguish movements and provenance of native fish in the Grand Canyon. River Res. Appl. 2013, 29, 1318–1329. [Google Scholar] [CrossRef]
- Wickström, H.; Sjöberg, N.B. Traceability of stocked eels—The Swedish approach. Ecol. Freshw. Fish 2014, 23, 33–39. [Google Scholar] [CrossRef]
- Stötera, S.; Degen-Smyrek, A.K.; Krumme, U.; Stepputtis, D.; Bauer, R.; Limmer, B.; Hammer, C. Marking otoliths of Baltic cod (Gadus morhua Linnaeus, 1758) with tetracycline and strontium chloride. J. Appl. Ichthyol. 2019, 35, 427–435. [Google Scholar] [CrossRef]
- Duffy, W.J.; McBride, R.S.; Hendricks, M.L.; Oliveira, K. Otolith age validation and growth estimation from oxytetracycline-marked and recaptured American shad. Trans. Am. Fish. Soc. 2012, 141, 1664–1671. [Google Scholar] [CrossRef]
- Frenkel, V.; Kindschi, G.; Zohar, Y. Noninvasive, mass marking of fish by immersion in calcein: Evaluation of fish size and ultrasound exposure on mark endurance. Aquaculture 2002, 214, 169–183. [Google Scholar] [CrossRef]
- Cottingham, A.; Hall, N.G.; Loneragan, N.R.; Jenkins, G.I.; Potter, I.C. Efficacy of restocking an estuarine-resident species demonstrated by long-term monitoring of cultured fish with alizarin complexone-stained otoliths. A case study. Fish. Res. 2020, 227, 105556. [Google Scholar] [CrossRef]
- Caraguel, J.-M.; Charrier, F.; Mazel, V.; Feunteun, E. Mass marking of stocked European glass eels (Anguilla anguilla) with alizarin red S. Ecol. Freshw. Fish 2015, 24, 435–442. [Google Scholar] [CrossRef]
- Simon, J.; Wickström, H. Long-term retention of alizarin red S marks and coded wire tags in European eels. Fish. Res. 2020, 224, 105453. [Google Scholar] [CrossRef]
- Braux, E.D.; Warren-Myers, F.; Dempster, T.; Fjelldal, P.G.; Hansen, T.; Swearer, S.E. Osmotic induction improves batch marking of larval fish otoliths with enriched stable isotopes. ICES J. Mar. Sci. 2014, 71, 2530–2538. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, S.H.; Gillanders, B.M.; Munro, A.R.; Crook, D.A.; Sanger, A.C. Determining mark success of 15 combinations of enriched stable isotopes for the batch marking of larval otoliths. N. Am. J. Fish. Manag. 2011, 31, 843–851. [Google Scholar] [CrossRef]
- Ennevor, B.C.; Beames, R.M. Use of lanthanide elements to mass mark juvenile salmonids. Can. J. Fish. Aquat. Sci. 1993, 50, 1039–1044. [Google Scholar] [CrossRef]
- Brown, P.; Green, C.; Sivakumaran, K.P.; Stoessel, D.; Giles, A. Validating otolith annuli for annual age determination of common carp. Trans. Am. Fish. Soc. 2004, 133, 190–196. [Google Scholar] [CrossRef]
- Swanson, R.G.; Gagnon, J.E.; Miller, L.M.; Dauphinais, J.D.; Sorensen, P.W. Otolith microchemistry of common carp reflects capture location and differentiates nurseries in an interconnected lake system of the North American Midwest. N. Am. J. Fish. Manag. 2020, 40, 1100–1118. [Google Scholar] [CrossRef]
- Smith, B.b.; Walker, K.F. Validation of the aging of 0+ carp (Cyprinus carpio L.). Mar. Freshw. Res. 2003, 54, 1005–1008. [Google Scholar] [CrossRef]
- Pan, X.D.; Ye, Z.J.; Xu, B.D.; Jiang, T.; Yang, J.; Tian, Y.J. Population connectivity in a highly migratory fish, Japanese Spanish mackerel (Scomberomorus niphonius), along the Chinese coast, implications from otolith chemistry. Fish. Res. 2020, 231, 105690. [Google Scholar] [CrossRef]
- Santos, R.O.; Schinbeckler, R.; Viadero, N.; Larkin, M.F.; Rennert, J.J.; Shenker, J.M.; Rehage, J.S. Linking bonefish (Albula vulpes) populations to nearshore estuarine habitats using an otolith microchemistry approach. Environ. Biol. Fishes 2019, 102, 267–283. [Google Scholar] [CrossRef]
- Yang, J.; Arai, T.; Liu, H.; Miyazaki, N.; Tsukamoto, K. Reconstructing habitat use of Coilia mystus and Coilia ectenes of the Yangtze River estuary, and of Coilia ectenes of Taihu Lake, based on otolith strontium and calcium. J. Fish Biol. 2006, 69, 1120–1135. [Google Scholar] [CrossRef]
- Bostanci, D. Otolith biometry-body length relationships in four fish species (chub, pikeperch, crucian carp, and common carp). J. Freshw. Ecol. 2009, 24, 619–624. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, T.; Yang, J. Unravelling habitat use of Coilia nasus from the Rokkaku River and Chikugo River estuaries of Japan by otolith strontium and calcium. Acta Oceanol. Sin. 2018, 37, 52–60. [Google Scholar] [CrossRef]
- Morioka, S.; Matsumoto, S. Otolith features and utility of lapillus for daily increment analysis in Opsaridium microcephalum (Cyprinidae) juveniles collected from Lake Malawi. Ichthyol. Res. 2003, 50, 82–85. [Google Scholar] [CrossRef]
- Morioka, S.; Matsumoto, S.; Kaunda, E. Otolith features and growth of Malawian characid Hemigrammopetersius barnardi from the southwestern coast of Lake Malawi. Ichthyol. Res. 2006, 53, 143–147. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Farina, M.; Ludka, I.P.; Kachar, B. Vaterite, calcite, and aragonite in the otoliths of three species of piranha. Naturwissenschaften 1996, 83, 133–135. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Ladich, F.; Plath, M.; Heß, M. Enigmatic ear stones: What we know about the functional role and evolution of fish otoliths. Biol. Rev. Camb. Philos. Soc. 2019, 94, 457–482. [Google Scholar] [CrossRef]
- Ren, D.; Meyers, M.A.; Zhou, B.; Feng, Q. Comparative study of carp otolith hardness: Lapillus and Asteriscus. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1876–1881. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Swearer, S.E.; Kapp, E.A.; Peng, P.; Tonkin-Hill, G.Q.; Papenfuss, A.; Roberts, A.; Bernard, P.; Roberts, B.R. The inner ear proteome of fish. FEBS J. 2019, 286, 66–81. [Google Scholar] [CrossRef]
- Mugiya, Y.; Tanaka, S. Incorporation of waterborne strontium into otoliths and its turnover in the goldfish Carassius auratus: Effects of strontium concentrations, temperature, and 17β-estradiol. Fish. Sci. 1995, 61, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Mugiya, Y.; Satoh, C. Strontium accumulation in slow-growing otoliths in the goldfish Carassius auratus. Fish. Sci. 1997, 63, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Hüssy, K.; Mosegaard, H. Atlantic cod (Gadus morhua) Growth and otolith accretion characteristics modelled in a bioenergetics context. Can. J. Fish. Aquat. Sci. 2004, 61, 1021–1031. [Google Scholar] [CrossRef]
- Wright, P.J.; Fallon-Cousins, P.; Armstrong, J.D. The relationship between otolith accretion and resting metabolic rate in juvenile Atlantic salmon during a change in temperature. J. Fish Biol. 2001, 59, 657–666. [Google Scholar] [CrossRef]
- Brown, P.; Harris, J.H. Strontium batch marking of golden perch (Macquaria ambigua Richardson) (Percichthyidae) and trout cod (Maccullochella macquariensis) (Cuvier). In Recent Developments in Fish Otolith Research; Secor, D.H., Dean, J.M., Campana, S.E., Eds.; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 693–702. [Google Scholar]
- Yokouchi, K.; Fukuda, N.; Shirai, K.; Aoyama, J.; Daverat, F.; Tsukamoto, K. Time lag of the response on the otolith strontium/calcium ratios of the Japanese eel, Anguilla japonica to changes in strontium/calcium ratios of ambient water. Environ. Biol. Fish. 2011, 92, 469–478. [Google Scholar] [CrossRef]
- Panfili, J.; Darnaude, A.M.; Vigliola, L.; Jacquart, A.; Labonne, M.; Gilles, S. Experimental evidence of complex relationships between the ambient salinity and the strontium signature of fish otoliths. J. Exp. Mar. Biol. Ecol. 2015, 467, 65–70. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; Johnson, R.C.; Waring, C.P.; Trueman, C.N. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 6, 806–816. [Google Scholar] [CrossRef]






| Sample Cord | First Phase | Second Phase | Third Phase | |||
|---|---|---|---|---|---|---|
| Distance from the Core (μm) | Sr/Ca (Mean ± SD) | Distance from the Core (μm) | Sr/Ca (Mean ± SD) | Distance from the Core (μm) | Sr/Ca (Mean ± SD) | |
| 20-1-L | 0–406 | 3.03 ± 0.59 b | 408-426 | 37.65 ± 16.30 a | 428–440 | 2.92 ± 0.31 b |
| 20-2-L | 0–440 | 3.09 ± 0.56 b | 442–458 | 32.12 ± 22.61 a | 460–474 | 3.18 ± 0.64 b |
| 20-3-L | 0–364 | 3.01 ± 0.67 b | 366–384 | 38.74 ± 21.75 a | 386–396 | 2.71 ± 0.54 b |
| 20-4-L | 0–402 | 3.04 ± 0.67 b | 404–422 | 38.46 ± 21.89 a | 424–432 | 3.23 ± 0.55 b |
| 20-5-L | 0–384 | 3.09 ± 0.62 b | 386–408 | 32.26 ± 21.06 a | 410–422 | 3.01 ± 0.35 b |
| Average | - | 3.05 ± 0.04 B | - | 35.85 ± 3.36 A | - | 3.01 ± 0.21 B |
| 20-1-S | 0–358 | 3.78 ± 0.57 b | 360–378 | 38.23 ± 21.54 a | 380–386 | 3.66 ± 0.23 b |
| 20-2-S | 0–340 | 3.80 ± 0.52 b | 342–356 | 39.06 ± 27.51 a | 358–364 | 4.01 ± 0.46 b |
| 20-3-S | 0–286 | 3.82 ± 0.57 b | 288–302 | 41.64 ± 23.74 a | 304–312 | 3.89 ± 0.93 b |
| 20-4-S | 0–370 | 3.78 ± 0.54 b | 372–386 | 33.02 ± 23.17 a | 388–394 | 4.01 ± 0.36 b |
| 20-5-S | 0–338 | 3.79 ± 0.62 b | 340–350 | 36.17 ± 25.03 a | 352–360 | 3.40 ± 0.22 b |
| Average | - | 3.79 ± 0.02 A | - | 37.62 ± 3.24 A | - | 3.79 ± 0.06 A |
| 20-1-A | 0–382 | 1.01 ± 0.41 b | 384–392 | 8.22 ± 2.72 a | 394–402 | 1.00 ± 0.79 b |
| 20-2-A | 0–296 | 1.09 ± 0.59 b | 298–326 | 6.86 ± 2.66 a | 328–346 | 1.61 ± 0.64 c |
| 20-3-A | 0–384 | 1.08 ± 0.43 b | 386–392 | 9.04 ± 3.05 a | 394–396 | 1.79 ± 0.04 c |
| 20-4-A | 0–320 | 1.06 ± 0.58 b | 322–326 | 7.89 ± 2.81 a | 328–330 | 1.67 ± 0.51 b |
| 20-5-A | 0–386 | 1.04 ± 0.28 b | 388–394 | 9.07 ± 1.89 a | 396–398 | 1.94 ± 1.63b |
| Average | - | 1.06 ± 0.03 C | - | 7.95 ± 0.79 B | - | 1.42 ± 0.38 C |
| Sample Cord | Distance from the Core (μm) | Sr/Ca (Mean ± SD) |
|---|---|---|
| 20-1-L-0 | 0–434 | 3.18 ± 0.53 |
| 20-2-L-0 | 0–398 | 3.10 ± 0.50 |
| 20-3-L-0 | 0–386 | 3.05 ± 0.57 |
| 20-4-L-0 | 0–410 | 3.12 ± 0.55 |
| 20-5-L-0 | 0–370 | 3.06 ± 0.50 |
| Average | - | 3.10 ± 0.05 B |
| 20-1-S-0 | 0–268 | 3.61 ± 0.71 |
| 20-2-S-0 | 0–314 | 3.57 ± 0.69 |
| 20-3-S-0 | 0–304 | 3.61 ± 0.52 |
| 20-4-S-0 | 0–278 | 3.53 ± 0.52 |
| 20-5-S-0 | 0–286 | 3.61 ± 0.57 |
| Average | - | 3.59 ± 0.04 A |
| 20-1-A-0 | 0–398 | 1.09 ± 0.32 |
| 20-2-A-0 | 0–370 | 1.12 ± 0.45 |
| 20-3-A-0 | 0–380 | 1.11 ± 0.37 |
| 20-4-A-0 | 0–356 | 1.10 ± 0.30 |
| 20-5-A-0 | 0–440 | 1.09 ± 0.33 |
| Average | - | 1.10 ± 0.01 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Jiang, T.; Chen, X.; Liu, H.; Phelps, Q.; Yang, J. Inter-Otolith Differences in Strontium Markings: A Case Study on the Juvenile Crucian Carp Carassius carassius (Linnaeus, 1758). Fishes 2022, 7, 112. https://doi.org/10.3390/fishes7030112
Zhu Y, Jiang T, Chen X, Liu H, Phelps Q, Yang J. Inter-Otolith Differences in Strontium Markings: A Case Study on the Juvenile Crucian Carp Carassius carassius (Linnaeus, 1758). Fishes. 2022; 7(3):112. https://doi.org/10.3390/fishes7030112
Chicago/Turabian StyleZhu, Yahua, Tao Jiang, Xiubao Chen, Hongbo Liu, Quinton Phelps, and Jian Yang. 2022. "Inter-Otolith Differences in Strontium Markings: A Case Study on the Juvenile Crucian Carp Carassius carassius (Linnaeus, 1758)" Fishes 7, no. 3: 112. https://doi.org/10.3390/fishes7030112