Abstract
Fungi are generally thought to live in host plants with a single lifestyle, being parasitism, commensalism, or mutualism. The former, known as phytopathogenic fungi, cause various plant diseases that result in significant losses every year; while the latter, such as endophytic fungi, can confer fitness to the host plants. It is unclear whether biological factors can modulate the parasitic and mutualistic traits of a fungus. In this study, we isolated and characterized a mycovirus from an endophytic strain of the fungus Pestalotiopsis theae, a pathogen of tea (Camellia sinensis). Based on molecular analysis, we tentatively designated the mycovirus as Pestalotiopsis theae chrysovirus-1 (PtCV1), a novel member of the family Chrysoviridae, genus Alphachrysovirus. PtCV1 has four double-stranded (ds) RNAs as its genome, ranging from 0.9 to 3.4 kbp in size, encapsidated in isometric particles. PtCV1 significantly reduced the growth rates of its host fungus in vitro (ANOVA; P-value < 0.001) and abolished its virulence in planta (ANOVA; P-value < 0.001), converting its host fungus to a non-pathogenic endophyte on tea leaves, while PtCV1-free isolates were highly virulent. Moreover, the presence of PtCV1 conferred high resistance to the host plants against the virulent P. theae strains. Here we report a mycovirus that modulates endophytic and phytopathogenic fungal traits and provides an alternative approach to biological control of plant diseases caused by fungi.
Similar content being viewed by others
Introduction
Fungi are generally considered to adopt a single symbiotic lifestyle, either parasitism, commensalism, or mutualism, during their interactions with host plants in nature. Plant parasitic fungi, also known as phytopathogenic fungi, affect plant growth rates and/or fecundity, and cause various diseases resulting in significant economic losses every year. Commensal fungi co-exist with plants with no apparent detrimental or beneficial effects for the host. Fungi that form mutualistic relationships with their plant hosts, such as arbuscular mycorrhizae associated with plant roots, may enhance nutrient acquisition, confer abiotic and biotic stress tolerance, increase growth and biomass, and decrease water consumption of the host plant. Endophytic fungi ubiquitously grow within plants and may range from harmful to asymptomatic to beneficial [1,2,3,4,5,6,7].
Pestalotiopsis spp., belonging to the family Amphisphaeriaceae, have a wide geographical distribution throughout tropical and temperate regions. They are common phytopathogens that reduce production and cause economic losses in fruit, tea, flower, and forest trees. Infection symptoms include canker lesions, shoot dieback, leaf spots, tip blight and fruit rots [8]. Pestalotiopsis theae is the causative agent of gray blight disease or brown-black spot disease in tea plants (Camellia sinensis (L.) O. Ktze.), resulting in a yield loss of over 10% yearly [9]. Pestalotiopsis spp. are also commonly found as non-pathogenic endophytes, producing novel compounds with medicinal, agricultural, and industrial applications [8]. Sequence and phylogenetic analyses revealed there are no significant differences at the molecular level between pathogenic and non-pathogenic species; therefore, it is speculated that their lifestyles are linked to host physiology and environmental conditions.
Mycoviruses, which have been reported in all phyla of fungi, possess double-stranded (ds) RNA, positive-sense (+) single-stranded (ss) RNA, negative-sense (−) ssRNA or ssDNA genomes [10] and are currently classified in 20 taxa. One of these taxa, the Chrysoviridae family, has two genera Alphachrysovirus and Betachrysovirus, accommodating mycoviruses with dsRNA genomes individually encapsidated in non-enveloped isometric particles ca. 40 nm in diameter [11]. Chrysoviruses infect ascomycetous or basidiomycetous fungi, plants and possibly insects, are transmitted both vertically and horizontally, and are typically associated with latent infections [11].
Similarly to the symbiosis between a fungus and its host plant, the relationship between a mycovirus and its host fungus can be mutualistic, commensal, or parasitic. Some mycoviruses can attenuate the virulence of their fungal hosts in plants, and therefore have been used as biological control agents for phytopathogenic fungi. For instance, Cryphonectria hypovirus 1 (CHV-1), a (+)ssRNA virus belonging to the family Hypoviridae [12], induces hypovirulence in severe strains of Cryphonectria (Endothia) parasitica, the causative agent of chestnut blight disease [13,14,15]. Similarly, Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1), a ssDNA virus belonging to the family Genomoviridae [16], efficiently controls the white mold S. sclerotiorum [17].
To date, a few studies have reported the presence of mycoviruses in endophytic fungi infecting grasses [18, 19]. Mycoviruses have been associated with plant adaptation to extreme environments, conferring heat tolerance to plants that contain fungal endophytes [20]. Nevertheless, further investigation is needed to establish whether mycoviruses may be responsible for the endophytic traits of their host fungus and whether they could be used in biological control applications. For example, the mycovirus-mediated endophytic state of fungi that confer resistance against biotic and abiotic stress to their plant host would serve as an alternative approach to plant protection and benefit crop production.
Here, we sought to investigate a mycovirus, Pestalotiopsis theae chrysovirus-1 (PtCV1), and its effects on the P. theae endophytic strain from which it was isolated. We report that PtCV1 has four dsRNA segments as its genome, each one individually encapsidated in isometric virions. We demonstrate by completing Koch’s postulates that the presence of PtCV1 is responsible for the diminished growth rates and the endophytic traits of the strain under study. PtCV1 is a mycovirus that can convert a destructive fungus into a non-pathogenic endophyte; the latter conferring disease resistance to the host plant against the former, illustrating the potential of PtCV1 as a biological control agent.
Materials and methods
Fungal strains and virus eradication
P. theae strain TP2-2W (accession no. KM513607) was isolated from a tea leaf showing the typical symptoms of tea gray blight disease collected in Yichang, Hubei province, China [21]. P. theae strain LI41 was isolated from a healthy tea leaf collected in Xuan’en county, En’shi prefecture, Hubei province, China. In addition to morphological identification, molecular identification was performed as described previously [21], revealing that the ribosomal internal transcribed spacer (ITS, including a partial 18S ribosomal RNA gene, internal transcribed spacer 1, 5.8S ribosomal RNA gene, internal transcribed spacer 2, and partial 28S ribosomal RNA gene) sequence of LI41 (accession no. MF115525) is identical to that of P. theae strain TP-1-1O characterized in our previous study [21]. P. theae strain L141 was found to harbor a quadripartite mycovirus nominated Pestalotiopsis theae chrysovirus 1 (PtCV1) and the PtCV1-free P. theae strain L141-1 was obtained from L141 following single conidia isolation. All strains were grown at 25 °C in the dark for 5–8 days on solid potato dextrose agar (PDA; 20% diced potatoes, 2% glucose, and 1.5% agar) unless stated otherwise. Strain gL141 was a subisolate of L141 labeled with green fluorescent protein (GFP) as described previously [22].
dsRNA extraction and purification
For dsRNA extraction, mycelial plugs of P. theae strain L141 were inoculated onto sterilized cellophane disks on PDA plates. Total nucleic acids were isolated from mycelia as described previously [23] and eluted with RNase-free water prior to enzymatic digestion of fungal RNA and DNA. Aliquots of 200 ng nucleic acids were treated with 2 U DNase I (New England Biolabs) and 10 U S1 nuclease (Thermo Scientific) at 37 °C for 1 h. The PtCV1 dsRNA was extracted with phenol/chloroform/isoamyl alcohol (25:24:1) using water saturated phenol (pH 5.2) and precipitated with ethanol at −20 °C overnight. The resultant pellets obtained by centrifugation were dried and dissolved in diethyl pyrocarbonate (DEPC)-treated water. The PtCV1 dsRNAs were fractionated by electrophoresis on 1.2% agarose gels with Tris-acetate-EDTA (TAE) buffer and visualized by staining with ethidium bromide. Each of the four PtCV1 genomic dsRNAs was excised, purified using a gel extraction kit (Qiagen, USA), dissolved in DEPC-treated water and stored at −70 °C until use.
Cloning, sequencing, and sequence analysis
The sequences of the four PtCV1 genomic dsRNAs were determined by cloning and sequencing amplicons generated by reverse transcription and polymerase chain reaction (RT-PCR) using the random primers 05RACE-3RT and 05RACE-3 (Table S1) as previously described [21]. The 5′- and 3′-terminal sequences of the dsRNAs were obtained by cloning and sequencing the RT-PCR amplicons generated using a standard RNA ligase mediated rapid amplification of cDNA ends (RLM-RACE) protocol (Table S1). The oligonucleotide primers used for RLM-RACE were designed based on sequence information obtained from the randomly primed amplicons [24]. At least three independent clones of each amplicons were sequenced in both directions, by Sangon Biotech Co., Ltd, Shanghai, China.
Sequence similarity searches were performed using BLASTN program for nucleic acids or BLASTP for putative proteins against the National Center for Biotechnology Information (NCBI) databases. Multiple alignments of nucleic and amino acid sequences were conducted using MAFFT version 6.85, as implemented at http://www.ebi.ac.uk/Tools/msa/mafft/ with default settings. The phylogenetic tree for RdRp sequences was constructed using MEGA 6 with Maximum Likelihood method [25]. RdRp sequences were aligned with MUSCLE as implemented by MEGA 6 [25], all positions with less than 30% site coverage were eliminated and the LG + G + I + F substitution model was used. Open reading frames (ORFs) were deduced using ORFfinder (https://www.ncbi.nlm.nih.gov/orffinder/).
Virus purification
For virus purification, mycelial plugs of P. theae strain L141 were inoculated onto sterilized cellophane disks on PDA plates. Mycelia were harvested and ground to a fine powder in liquid nitrogen and extracted as previously described [26]. Briefly, ca. 30 g mycelia were mixed with 100 mM phosphate buffer (PB; 8.0 mM Na2HPO4, 2.0 mM NaH2PO4, pH 7.0) and centrifuged at 12,096×g at 4 °C for 30 min to remove cellular debris. The supernatant was then ultracentrifuged (Optima LE-80K; Beckman Coulter, Inc.) at 110,000×g at 4 °C for 2 h to collect the virus pellet, which was resuspended in 100 mM PB buffer. The crude virus preparation was purified further by sucrose gradient centrifugation [26]. Subsequently aliquots of each fraction (100 μL) were subjected to dsRNA extraction to monitor for the presence of viral dsRNAs. Crude and purified virus preparations were negatively stained with 1% uranyl acetate on carbon-coated 400-mesh copper grids and examined by transmission electron microscopy (TEM; H-7000FA; Hitachi). The inner and outer widths of the virions were measured using Image J 1.43 [27].
SDS-polyacrylamide gel electrophoresis and peptide mass fingerprinting
Proteins extracted from each sucrose gradient fraction were fractionated by 12% SDS-polyacrylamide gel electrophoresis (PAGE) in a 25-mM Tris/glycine and 0.1% SDS buffer. Gels were stained with Coomassie brilliant blue R-250 (Bio-Safe CBB; Bio-Rad, USA), and protein bands were individually excised and subjected to peptide mass fingerprinting (PMF) analysis [28] by Sangon Biotech, Co., Ltd, Shanghai China.
Contact cultures of P. theae isolates
Horizontal transmission of PtCV1 originally isolated from P. theae strain L141 was assessed as previously [29]. P. theae strains L141 (PtCV1-infected; donor) and L141-1 (PtCV1-free; recipient) were cultured together on 9 cm diameter Petri dishes at 25 °C for 7 days and allowed to physically contact each other. Following contact, mycelial agar plugs from the colony margin of L141-1 were subcultured onto fresh PDA plates. Ten independent donor-recipient pairs were assessed and four mycelial agar plugs were selected from each pair for further analysis, resulting in a total of 40 isolates.
Protoplast transfection with dsRNAs and virions
Protoplasts were isolated from conidia derived from actively growing mycelia of the PtCV1-free P. theae strain L141-1. Isolated protoplasts were filtered through a Millipore filter and counted under a microscope using a hemocytometer; 2.0 × 106 protoplasts were used for transfection with ca. 5.0–7.0 μg PtCV1 dsRNA or 70.0–80.0 μg PtCV1 virions in the presence of PEG 6000 as previously described [30]. Following transfection protoplast suspensions were diluted with sterilized water, spread onto PDA plates and fungal colonies allowed to regenerate prior to evaluation of PtCV1-infected status.
Growth rate, virulence and challenge inoculation assays
Individual disks (5 mm in diameter) of P. theae mycelia grown on PDA were taken from the edge of growing colonies using a sterile puncher and placed in the center of fresh PDA plates. Colony diameters were measured daily up to 4 days post inoculation (dpi) using the cross intersect method subtracting the diameter of the original disc. Six biological replicates for each strain were monitored and the results subjected to statistical analysis as described below.
The virulence of individual P. theae strains was determined following inoculation of detached tea leaves (C. sinensis vars. Guilv no.1, Tieguanyin, Yingshuang, Wuniuzao, and Fudingdahao) using a modified version of a published protocol [21]. Briefly, detached tea leaves were washed three times with sterile water and air-dried, prior to inoculation. Disks of P. theae mycelia were prepared as described above and placed in the middle of the adaxial surface of detached tea leaves that were wounded three times with a needle (insect pin, 0.45 mm in diameter). After inoculation, the detached tea leaves were put on plastic trays, covered with plastic wrap to maintain a 99% relative humidity, and incubated in a climate chamber at 25 °C with a 12/12 h light/dark photoperiod. At 6 dpi, lesions that developed on the inoculated leaves were measured. Six biological replicates for each strain were monitored and the results subjected to statistical analysis as described below.
For the challenge inoculation assays, the mycelial disks of the PtCV1-infected P. theae strain LI41-1T1 were removed 2 dpi, and mycelial disks of the PtCV1-free P. theae strain LI41-1 were placed on the tea leaves, either at the same position, or at a different position close to the tip, or on other leaves of the same branches, after wounding three times with a needle. Non-inoculated PDA disks were used in parallel as controls. The leaves were then incubated as above and any necrotic lesions that developed were measured and photographed 9 dpi. In total, 15–21 biological replicates were monitored for each treatment and the results were subjected to statistical analysis as described below.
Confocal scanning laser microscopy of tea leaf tissue
Intact tea leaves (C. sinensis var. Tieguanyin) were inoculated with either the P. theae virus-free isolate or virus-infected isolate as described above. Asymptomatic tissue disks (5 mm in diameter) were excised from positions approximately 10 mm away from respectively lesions or inoculation sites at 15 dpi. Tissue disks were then fixed in vacuo with 4% paraformaldehyde in 1×PBS and washed with 1×PBS; 40-µm-thick sections were cut using a Vibratome (Leica VT1200S, Bensheim) as described previously [31]. Leaf sections were then stained with WGA-AF488 (Invitrogen, Thermo Fisher Scientific) which stains fungal mycelia green following excitation at 488 nm and detection at 505–550 nm, or with propidium iodide (PI; Invitrogen, Thermo Fisher Scientific) which stains plant cells red following excitation at 552 nm and detection at 590–640 nm. Stained cells were visualized by confocal laser scanning microscopy (Leica SP8, Bensheim).
Statistical analyses
For the growth rate, virulence and challenge inoculation assays, the mean values for the biological replicates are presented as column charts with error bars representing standard deviation (SD), while the individual values are presented as blue dots. The graphs were produced in Excel (Microsoft) and GraphPad Prism 7 (GraphPad software). Identification of potential outliers, Shapiro–Wilk normality test (n = 4), Kolmogorov–Smirnov normality test (n > 4), Levene’s test for homogeneity of variances, one-way analysis of variance (ANOVA) and Games-Howel post-hoc tests were performed using IBM SPSS Statistics. Briefly, the normality tests indicated that all data sets were well-modeled by a normal distribution, therefore one-way ANOVA was performed to assess statistically significant overall differences. Levene’s test indicated that homogeneity of variances should not be assumed, therefore Games-Howel post-hoc tests were performed to assess statistically significant pairwise differences. Outliers, when present, did not affect the outcome of the statistical analysis. P-values < 0.05 were considered to indicate statistical significance.
Results and discussion
A complex pattern of dsRNAs in P. theae strain LI41
Nucleic acid preparations enriched in dsRNA were obtained from P. theae strain LI41 isolated from a tea leaf collected in China, and were subjected to digestion with DNase I and S1 nuclease and then agarose gel electrophoresis. The results showed that four dsRNAs (nominated 1–4 according to their decreasing sizes) were detected in preparations of strain LI41 but not in a pathogenetic strain, e.g. TP2-2W (Fig. 1A). The sequences of the full-length cDNAs of dsRNAs 1–4 were determined by assembling partial-length cDNAs that were amplified from the purified dsRNAs using RT-PCR and RLM-RACE protocols. The corresponding sequences were deposited in GenBank with accession numbers MH323409- MH323412. To our knowledge, this is the first report of dsRNA elements in Pestalotiopsis spp.
a Electrophoretic profiles on a 1.2% agarose gel of dsRNA preparations from strain LI41 before (−) and after (+) digestion with DNase I and S1 nuclease and from strain TP2-2W without digestion with either enzyme. b Schematic representation of the genomic organization of PtCV1 dsRNAs 1–4 showing putative ORFs as gray boxed and UTRs as black lines.
dsRNAs 1 to 4 compose the genome of a novel alphachrysovirus
The sequences of the four dsRNAs comprised 3391, 2798, 2599, and 932 base pairs (bp), respectively, with each containing a single ORF in the positive strand encoding putative proteins with estimated molecular mass (Mr) values of 126, 102, 93, and 18 kDa, respectively (Fig. 1B).
The 5′-UTRs of the coding strands of the dsRNAs are respectively 61, 64, 66 and 35 bp in length (Fig. 1B) and share 48.6–84.4% identity, whereas the corresponding 3′-UTRs are 50, 28, 52 and 417 bp long (Fig. 1B) and share 18.6–68.0 % identity. The 5′- and 3′- UTRs of dsRNAs 1, 2, and 3 are conserved and contain identical or almost identical regions, 57 bp at the former (5′-GAU AAA AAU ACA AAA CGC [U/A]UU UUU AUC CUG AGU GA[A/C] GUU GAC ACA U[U/A]A AAC AAC [U/G]C-3′) and 8 bp (5′-UUA AGC GU-3′) at the latter (Fig. 2A). However, the conserved 5′-terminus of dsRNA 4 is 36 bp shorter than those of dsRNAs 1–3 (Fig. 2A).
a Conserved sequences of the 5′-termini (top) and 3′-termini (bottom) of the dsRNAs 1–4. Black, gray and light gray backgrounds denote nucleotide identity of no less than 100%, 75%, and 50%, respectively. b ML phylogenetic tree created based on the RdRp sequences of PtCV1 and all the members in the family Chrysoviridae, as listed in Table S4. The percentage of trees in which the associated taxa clustered together is shown next to the branches, and the number in brackets after the virus name indicates the number of dsRNA genomic segments. Saccharomyces cerevisiae virus L-A, a member of the genus Totivirus, family Totiviridae, was used as the outgroup.
BLASTP searches of the amino acid sequences of ORFs 1–3 predicted from the sequences of dsRNAs 1–3 revealed highest identities (65%, 48%, and 54%) with the RdRp, coat protein (CP) and putative protease of Colletotrichum gloeosporioides chrysovirus 1 (CgCV1; [32]) (Table S2). ORF4 putatively encoded by dsRNA 4 shares no detectable identity with any known viral proteins, whereas there was moderate identity with hypothetical proteins encoded by Gammaproteobacteria (accession no. PKM23163, coverage 39%, E value 0.17, and identity 41%) and Gossypium barbadense (no. PPD81271, coverage 54%, E value 7.8, and identity 31%; Table S2). Phylogenetic analysis of the putative RdRp sequence encoded by ORF1 with the RdRps of selected members of the family Chrysoviridae illustrated that the sequence clustered with CgCV-1 RdRp in the Alphachrysovirus genus (Fig. 2B). Overall these results suggest that dsRNAs 1–4 are the genomic components of a novel alphachrysovirus which was named Pestalotiopsis theae chrysovirus-1 (PtCV1).
PtCV1 possessed several unique molecular characteristics absent from other members of the Chrysoviridae. For instance, the 5′-termini of PtCV1 dsRNAs 1–4 lack a stretch of CAA repeats [32,33,34] and the lengths of the 5′-UTRs of PtCV1 dsRNAs 1–4, whilst similar in size to those of CgCV1 (67–72 bp), are significantly shorter (15–66 bp) than those of other members of the Chrysoviridae (140–400 bp) [32, 35]. The total size of the PtCV1 genome (9716 bp) is small compared to most chrysoviruses (8.9–16.0 bp) [11] and PtCV1 dsRNA 4 (932 bp) is significantly smaller than the dsRNA 4 elements of all sequenced alphachrysoviruses and has no homology with them. PtCV1 is most closely related to CgCV-1, which is a trichrysovirus and has only three genomic components encoding the alphachrysovirus RdRp, CP and protease [32]. Perhaps PtCV1 should be considered a trichrysovirus as well, since it lacks the fourth dsRNA component encoding the hypothetical protein whose homologs are present in all alphachrycoviruses with four genomic segments. The existence of small dsRNA elements that encode non-homologous proteins of unknown function is well documented for members of the genus Betachysovirus, which may have up to three such dsRNAs in addition to the four main chrysovirus genomic components, but not for members of the genus Alphachysovirus. CgCV-1 and PtCV1 are the only trichysoviruses that infects fungi instead of plants [11].
dsRNAs 1–4 are encapsidated in isometric virions
To determine whether PtCV1 dsRNAs are encapsidated, purified virus preparations were fractionated by sucrose gradient (100–500 mg/mL and with an increment of 100 mg/mL), centrifuged and examined for the presence of dsRNA by agarose gel electrophoresis (Fig. 3A) and for virus protein by SDS-PAGE (Fig. 3B). These investigations revealed that the four PtCV1 dsRNAs co-fractionated with two protein bands having Mr values of 150 and 65 kDa and the highest titer in 500 mg/mL sucrose fraction (Fig. 3A). TEM of those fractions revealed the presence of isometric virus-like particles (VLPs) with inner diameters of 20.8–34.1 nm and outer diameters of 30.1–39.8 nm (Fig. 3C). Size variation of the encapsidated virions probably reflects encapsidation of individual genomic PtCV1 dsRNAs, a characteristic feature of all chrysoviruses [11, 32].
A Agarose gel electrophoresis of dsRNAs extracted from purified PtCV1 VLPs obtained from sucrose gradient fractions ranging from 100 to 500 mg/mL with an increment of 100 mg/mL. M, DNA size marker. B SDS-PAGE of proteins extracted from the aforementioned sucrose gradient fractions. M, protein molecular weight marker. C Representative TEM images of VLPs purified from P. theae strains LI41 (left) and LI41-1T1 (right).
The two proteins (termed p150 and p65) were purified and subjected to PMF analysis. These results showed that p150 generated a total of eight peptide fragments which matched the partial sequences encoded by PtCV1 ORFs 2 and 3 (Table S3) however p65 was unstable and degraded easily and its function is unresolved. Two major structural proteins encoded by PtCV1 dsRNAs 2 and 3 suggest an icosahedral T = 1 capsid consisting of 60 CP heterodimers, similar to Botryosphaeria dothidea chrysovirus 1 (BdCV1) [26], Botrytis porri RNA virus 1 (BpRV1) [28], and quadriviruses [36].
PtCV1 is transmitted both vertically and horizontally
To investigate potential vertical transmission of PtCV1 in P. theae strain L141, individual conidia were isolated from mycelia and cultured on PDA. A total of 42 single subisolates were randomly selected and analyzed for the presence of PtCV1 dsRNAs. The experiments showed 28/42 (67%) of the subisolates were infected with PtCV1 confirming efficient vertical transmission of the virus (Fig. S1A). This was ratified following RT-PCR amplification of fragments of PtCV1 dsRNAs 2 and 3 using a combination of oligonucleotide primers RNA2-F1/-R1 for dsRNA 2 and RNA3-F1/-R1 for dsRNA 3, respectively (Fig. S1B, C and Table S1). To investigate horizontal transmission of PtCV1, PtCV1-infected L141, and PtCV1-free L141-1 were dual-cultured and L141-1 mycelia far from the contact area were analyzed for the presence of PtCV1 dsRNAs. This experiment revealed that 6/40 (15%) of the subisolates (nominated L141-1P1 to −1P6) were infected by PtCV1 (Fig. S2A) confirming that the virus can be horizontally transmitted through hyphal fusion.
Fungal protoplasts are transfected with PtCV1 virions but not dsRNA
Initially, attempts were made to transfect protoplasts derived from PtCV1-free strain LI41-1 with PtCV1 dsRNAs purified from strain LI41 (following S1 nuclease and DNase I treatment). A total of 150 subisolates regenerated from the transfected protoplasts were randomly subjected to dsRNA extraction and electrophoretic analysis, revealing that no subisolates were infected by PtCV1 (Fig. S2B). We further transfected the protoplasts derived from PtCV1-free strain LI41-1 with PtCV1 virions purified from sucrose gradient centrifuge from strain LI41. This process resulted in five subisolates infected by PtCV1 (designated as LI41-1T1 to −1T5), out of the 150 subisolates analyzed in total (Fig. S2C). When subisolate LI41-1T1 was subjected to TEM analyses, isometric VLPs were visible in sucrose fractions (30–50%) after virus purification (Fig. 3C, right panel). To date, only dsRNA mycoviruses belonging to the family Polymycoviridae have been reported to be infectious as naked genomic dsRNAs [23, 37], whereas chrysoviruses have only been transfected successfully with virions [38] but not dsRNA, in agreement with the results of the present study.
PtCV1 controls the morphology, growth, and pathogenicity of its fungal host
The PtCV1-infected subisolates derived from single conidia of the original LI41 strain (LI41-V1 and -V2), horizontal transmission (LI41-1P1 to −1P3), and virion transfection (LI41-1T1 to −1T3), together with the cured subisolates (LI41-1 to −3), were compared to establish the effects of the PtCV1 on its host by completing the Koch’s postulates.
PtCV1 infection altered the morphology and growth rates of the P. theae subisolates: all the cured subisolates (LI41-1 to −3) showed a morphology similar to that of normal P. theae pathogenic strains, such as TP2-2W described previously [21], with growth rates of 5.0–9.0 mm/d; conversely, all the PtCV1-infected subisolates (LI41-V1 and -V2, LI41-1T1 to −1T3, and LI41-1P1 to −1P3), were similar to strain LI41, forming sectored colonies without concentric aerial rings and with growth rates of 0.5–2.8 mm/d (Fig. 4AB). The mean difference in growth rate between PtCV1-free and PtCV1-infected subisolates was found to be statistically significant (ANOVA; P-value <0.001). Additionally, the mean difference in growth rate of each individual PtCV1-free subisolate as compared to each individual PtCV1-infected subisolate was found to be statistically significant (Games-Howel post-hoc test; P-value < 0.01 at least).
A Representative morphology of the PtCV1-infected strain LI41, PtCV1-infected subisolates derived from single conidia (LI41-V1 and -V2), transfection (LI41-1T1 to -T3) and horizontal transmission (LI41-1P1 to -P3), and PtCV1-free subisolates (LI41-1 to −3). B Growth rates of the aforementioned subisolates; columns indicate the average growth rate of six independent cultures for each subisolate, error bars represent standard deviation and blue dots indicate individual measurements. The differences between PtCV1-infected and PtCV1-free subisolates are statistically significant (one-way ANOVA: P-value < 0.001; Games-Howel post-hoc test: P-value < 0.01 at least). C Representative symptoms on tea leaves (Camellia sinensis var. Guilv no.1) following inoculation with the aforementioned PtCV1-infected and PtCV1-free subisolates at 6 dpi. D Lesion lengths induced by inoculation with the aforementioned subisolates; columns indicate the average growth rate of four independent cultures for each subisolate, error bars represent standard deviation and blue dots indicate individual measurements. The differences between PtCV1-infected and PtCV1-free subisolates are statistically significant (one-way ANOVA: P-value < 0.001; Games-Howel post-hoc test: P-value < 0.01 at least).
A pathogenicity test on detached, wounded tea leaves (C. sinensis var. Guilv no.1) revealed that the cured subisolates induced large lesions (11.9-14.1 mm at 6 dpi), whereas the PtCV1-infected subisolates induced no lesion (Fig. 4CD). The mean difference in lesion length between PtCV1-free and PtCV1-infected subisolates was found to be statistically significant (ANOVA; P-value < 0.001). Additionally, the mean difference in lesion length of each individual PtCV1-free subisolate as compared to each individual PtCV1-infected subisolate was found to be statistically significant (Games-Howel post-hoc test; P-value < 0.01 at least), while there are no statistically significant differences in the lesion lengths when comparing PtCV1-free and PtCV1-infected subisolates to each other. Pathogenicity tests on other tea varieties (Tieguanyin, Yingshuang, Wuniuzao, and Fudingdahao) showed similar results, i.e. large lesions following inoculation by the cured subisolates and no lesions following inoculation by the PtCV1-infected subisolates, under the same conditions (Fig. S3).
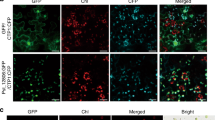
Further pathogenicity tests were contacted to compare the PtCV1-infected L141-1T1 and the PtCV1-free L141-1 isolates on intact tea plant leaves of C. sinensis cv. Tieguanyin under field conditions at 15 dpi. No symptoms were observed on leaves (0/10) following inoculation with L141-1T1 or in control inoculations, while necrotic lesions were obvious (10/10) following inoculation with L141-1 (Fig. 5AI). The presence of L141-1T1 in asymptomatic tissues and L141-1 exclusively in diseased tissues was confirmed by isolation of the fungus at 15 dpi. It total, 33 L141-1T1 colonies were recovered from 78 leaf disks (5 mm in diameter) collected ca. 10 mm from the inoculation sites. Similarly, 45/78 and 0/20 L141-1 colonies were recovered from the lesions and ca. 10 mm from the lesions, respectively. The identity of these colonies was confirmed by observing their morphology on PDA, which was initially similar (Fig. 5AII) and finally identical (after two or three rounds of subcultures) with that noted previously for 141-1T1 and L141-1, respectively, and by ITS sequencing. Correspondingly, PtCV1 dsRNA extraction from the recovered colonies illustrated the presence of the virus in the former and its absence in the latter (Fig. 5AIII). The presence of PtCV1 in the leaves was confirmed following diagnostic RT-PCR amplification of PtCV1 dsRNA 3 from RNA samples isolated from asymptomatic leaf tissue previously inoculated with L141-1T1 but not tissue inoculated with L141-1 (Fig. 5AIV). Asymptomatic leaf tissue ca. 10 mm from inoculation sites or lesions was excised and stained with WGA-AF488 and PI and examined using confocal laser scanning microscopy. L141-1T1 mycelia (green color) invaded leaf tissue (red color) without damaging it and that leaf cells remained intact similarly to PDA inoculated controls (Fig.5BI and II). In contrast, L141-1 mycelia broke down leaf tissue (Fig. 5BI) causing destruction of neighboring leaf cells around the damaged areas (Fig. 5BII).
A Representative symptoms on tea leaves (C. sinensis var. Tieguanyin) following inoculation with PtCV1-infected LI41-1T1 and PtCV1-free LI41-1; CK− indicates the negative control (I); representative LI41-1T1 and LI41-1 colonies isolated from the sites indicated by arrows in panel (I); agarose gel electrophoresis of dsRNA extracted from these colonies (III); and agarose gel electrophoresis of amplicons following RT-PCR of the extracted dsRNA with oligonucleotide primers specific for PtCV1 dsRNA3 (IV). B Confocal laser scanning microscopy of leaf tissues stained with WGA488/PI, and observed under bright field, red fluorescence and green fluorescence, together with the merged images (I); magnified merged images (II). Red and green fluorescence indicate plant and fungal tissues, respectively. Bar = 50 μm.
Completion of the Koch’s postulates shows conclusively that absence of PtCV1 converts endophytic P. theae strain L141 into a phytopathogen but re-introduction of PtCV1 into PtCV1-free isolates restores the non-pathogenic endophytic state. This phenomenon differs from the traditional, well-documented mycovirus-mediated hypovirulence: mycoviruses such as CHV-1 and SsHADV-1 [23,24,25, 27] simply reduce the virulence of their host fungus, leading to milder disease symptoms. The PtCV1-infected strains do not cause any disease symptoms at all but grow within the plant tissue as non-pathogenic endophytes.
The different symbiotic lifestyles of fungi are considered as a result of long-term evolution [39, 40] and usually depend on the host plants. For example, a Colletotrichum magna strain was shown to cause anthracnose in cucurbit, but to grow asymptomatically as an endophyte on an assortment of non-cucurbit species [41]. Plant physiology and environmental conditions may trigger a switching of lifestyle in fungi [42,43,44]. Previous studies provided evidence that nutrient status facilitates the transition of Colletotrichum tofieldiae from pathogenic to a beneficial lifestyle: under phosphorus-deficient conditions, C. tofieldiae transfers phosphorus to the shoots, promotes plant growth, and increases fertility of Arabidopsis thaliana [45]. The imbalance in nutrient exchange between plant and fungus is also responsible for switching from an endophytic to a parasitic lifestyle [46]. Moreover, lifestyle switching is linked to the genetic background of fungi or their host plants. For instance, a virulent C. magna isolate was converted into a non-pathogenic one, following treatment with ultraviolet light, and could asymptomatically colonize host plants conferring fitness [47]. C. tofieldiae had a severe negative effect on the growth of an A. thaliana cyp79B2 cyp79B3 double mutant, and eventually killed the plants under both high and low phosphorus conditions, indicating that in the absence of these two cytochrome P450 enzymes it becomes pathogenic [45]. However, transitions between different lifestyles mediated by biological factors, such as mycoviruses, under the same cultivation and environmental conditions, are far less documented.
PtCV1 confers resistance to host plants against pathogenic fungi
The interactions between the pathogenic and non-pathogenic P. theae strains were further investigated to understand how PtCV1 affects the interplay of the strains when both are found on the same tea plant. Tea leaves (C. sinensis var. Tieguanyin, n = 15) infected with non-pathogenic, PtCV1-infected LI41-1T1 were challenged by inoculation with pathogenic, PtCV1-free LI41-1 at 2 dpi. LI41-1 caused no symptoms on the leaves (0/15) infected with L141-1T1 at 9 dpi (Fig. 6AIII), similarly to the controls inoculated with PDA disks (Fig. 6AI). Conversely, the leaves (9/15) not previously inoculated with LI41-1T1 developed necrotic lesions (9.7–10.5 mm; Fig. 6AII).
A Representative symptoms on tea leaves (C. sinensis var. Tieguanyin) inoculated with PtCV1-free LI41-1 at 9 dpi, following pre-inoculation with uncolonized PDA (II) and PtCV1-infected LI41-1T1 (III) for 2 days. PDA indicates the negative control inoculated exclusively with uncolonized PDA disks (I). B representative LI41-1T1 and LI41-1 colonies isolated from sites indicated by arrows in panel A (I); representative confocal laser scanning microscopy pictures of leaf tissues stained with WGA488/PI, and observed under bright field, red fluorescence and green fluorescence, together with the merged images. Red and green fluorescence indicate plant and fungal tissues, respectively. Bar = 50 μm (II); agarose gel electrophoresis of dsRNA extracted from these colonies (III). C Representative symptoms on tea leaves (C. sinensis var. Tieguanyin) inoculated with gLI41-1 at 9 dpi, following pre-inoculation with uncolonized PDA (II) and PtCV1-infected LI41-1T3 (III) for 2 days. PDA indicates the negative control (I). D representative LI41-1T3 and LI41-1 colonies isolated from sites indicated by arrows in panel C (I); representative confocal laser scanning microscopy pictures of mycelia observed under bright field (II) and green fluorescence (III); and agarose gel electrophoresis of dsRNA extracted from these colonies (IV). E Lesion lengths induced by gLI41-1 following pre inoculation with PDA or LI41-1T3 on the same leaves (I), or LI41-1 following pre inoculation with PDA or LI41-1T1 on the neighboring leaves (II). Error bars represent standard deviation and blue dots indicate individual measurements. The stars indicate the significant differences between these treatments.
To ensure that the observation was not due to conversion of the PtCV1-free strain to PtCV1-infected by horizontal transmission of PtCV1 from LI41-1T1 to LI41-1, which were inoculated in the same position, the two strains were then inoculated in the bottom and tip of the tea leaves, respectively. Similar results were obtained: LI41-1 caused no symptoms on leaves (0/15) previously inoculated with L141-1T1 at 14 dpi, not previously inoculated with LI41-1T1 developed necrotic lesions (7.8–9.3 mm).
The presence of PtCV1-infected LI41-1T1 in the asymptomatic tissue around the inoculated sites was confirmed by isolation of the fungus and dsRNA extraction. In total, 15 PtCV1-infected LI41-1T1 colonies were recovered from 20 leaf disks collected ca. 0.5 cm from the inoculated sites (Fig. 6BI bottom, and II right). Correspondingly, the plant cells were intact and abundant fungal mycelia were observed inside the cells (Fig. 6BII bottom), as shown by confocal laser scanning microscopy. In contrast, 18/20 LI41-1 colonies were recovered from diseased tissue leaves and 0/20 LI41-1 colonies were recovered from the adjacent asymptomatic tissue ca. 1 cm far from the necrotic lesions (Fig. 6BI top). The recovered LI41-1 colonies contained no PtCV1 (Fig. 6BIII left) and plant cells were destroyed by the fungal invasion neighboring the inoculated sites (Fig. 6BII top).
To stringently exclude the possibility that the observed resistance is due to anastomosis and virus transmission between strains, PtCV1-free LI41-1 was labeled with GFP, and a transfectant (termed gLI41-1) without obvious changes in its morphology, growth rate and virulence as compared to the wild type, was chosen for challenge-inoculation experiments on tea leaves with PtCV1-infected LI41-1T3 as described above. The results were similar, i.e. gLI41-1 induced necrotic lesions (10.0–13.5 mm at 9 dpi, n = 16) following pre inoculation with PDA, while no lesions were noted following pre inoculation with LI41-1T3, similarly to the leaves inoculated exclusively with PDA (Fig. 6C, EI). Fungal isolation from the adjacent asymptomatic tissue (ca. 0.5 cm far from the inoculation sites) in the protected, pre inoculated leaves revealed 16 LI41-1T3 colonies (from 30 leaf disks) as confirmed by their morphology and dsRNA extraction (Fig. 6DI, IV, right panels). No gLI41-1 colonies were obtained as confirmed with GFP observation (Fig. 6DII, III, right panels). In contrast, 27 gLI41-1 colonies (from 30 leaf disks) were isolated from the diseased, challenge inoculated leaves as they expressed GFP (Fig. 6DI to III, left panels) and contained no PtCV1 dsRNAs (Fig. 6DIV). No fungal colonies were obtained in the control leaves inoculated exclusively with PDA.
To assess whether the observed resistance was systemic and could affect other leaves in addition to the ones directly inoculated with the PtCV1-infected LI41-1T1, PtCV1-free LI41-1 was used to challenge neighboring tea leaves on the same branches at 2 dpi. LI41-1 challenge inoculation led to no (12/21 leaves) or very small lesions (2.0–3.0 mm) on 9/21 leaves from plants pre inoculated with LI41-1T1, while big necrotic lesions (4.0–8.0 mm) were present on all leaves (27/27) from plants pre inoculated with PDA, revealing a clear resistance (Fig. 6EII).
These results indicate that the presence of PtCV1-infected, non-pathogenic, endophytic P. theae strains in planta protects against invasion and destruction of the plant tissue by pathogenic P. theae strains, illustrating a potential biological control mechanism based on the PtCV1-infected strain L141. A similar phenomenon of mycovirus-mediated resistance to disease was previously documented in oilseed rape (Brassica napus) with two closely related pathogenic fungi causing phoma stem canker, Leptosphaeria maculans and L. biglobosa, and Sclerotinia sclerotiorum causing sclerotinia stem rot [48,49,50]. In contrast to PtCV1 and SsHADV-1, infection with Leptosphaeria biglobosa quadrivirus 1 (LbQV1) resulted in hypervirulence of its host fungus;[48,49,50] nevertheless, the presence of PtCV1, SsHADV-1 and LbQV1 in their respective fungal hosts leads to plant resistance against more aggressive fungi. In the future, the effects of PtCV1 will be investigated in other P. theae strains to determine whether the phenomena described in the present work are strain-specific or have wider biological relevance within the fungal population.
In summary, many mycoviruses have been identified that modulate the virulence of phytopathogenic fungi [10, 51], but little is known about their roles in endophytic fungi apart from their previously reported ability to confer heat tolerance to the plant host [20]. Here we describe a mycovirus responsible for the endophytic traits of its host, mediating its lifestyle transition from a pathogenic to a non-pathogenic, endophytic fungus and establishing the latter as an excellent biological control agent against the former in the host plant. Our work illustrates how mycoviruses may influence the complex parasitic and mutualistic interactions between fungi and their host plants.
References
Arnold AE, Mejia LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, et al. Fungal endophytes limit pathogen damage in a tropical tree. Proc Natl Acad Sci USA 2003;100:15649–54.
Stone JK, Bacon CW, White J. An overview of endophytic microbes: endophytism defined. In: Bacon CW, White JF, editors. Microbial endophytes. New York: Marcel Decker Inc; 2000.
Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–30.
Bonfante P, Genre A. Mechanisms underlying beneficial plantfungus interactions in mycorrhizal symbiosis. Nat Commun 2010;1:48.
Abdelaziz M, Kim D, Ali S, Fedoroff N, Al-Babili S. The endophytic fungus piriformospora indica enhances arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci 2017;263:107–15.
Yadav A, Yadav K. Exploring the potential of endophytes in agriculture: a minireview. Adv Plants Agric Res 2017;6:102–6.
Vega FE. The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 2018;110:4–30.
Maharachchikumbura SSN, Hyde KD, Grenewald JZ, Xu J, Crous PW. Pestalotiopsis revisited. Stud Mycol. 2014;79:121–86.
Liu F, Hou L, Raza M, Cai L. Pestalotiopsis and allied genera from Camellia, with description of 11 new species from China. Sci Rep. 2017;7:866.
Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology 2015;479-480:356–68.
Kotta-Loizou I, Castón JR, Coutts RHA, Hillman BI, Jiang D, Kim D-H, et al. ICTV virus taxonomy profile: Chrysoviridae. J Gen Virol 2020;101:143–4.
Suzuki N, Ghabrial SA, Kim K, Pearson M, Marzano SL, Yaegashi H, et al. ICTV virus taxonomy profile: Hypoviridae. J Gen Virol 2018;99:615–6.
Anagnostakis SL. Biological control of chestnut blight. Science 1982;215:466–71.
MacDonald W, Fulbright D. Biological control of chestnut blight: use and limitations of transmissible hypovirulence. Plant Dis 1991;75:656–61.
Nuss DL. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol Mol Biol Rev. 1992;56:561–76.
Bamford DH, Pietilä MK, Roine E, Atanasova NS, Dienstbier A, Oksanen HM, et al. ICTV virus taxonomy profile: Pleolipoviridae. J Gen Virol 2017;98:2916–7.
Yu X, Li B, Fu Y, Xie J, Cheng J, Ghabrial SA, et al. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc Natl Acad Sci USA 2013;110:1452–7.
Herrero N, Márquez S, Zabalgogeazcoa I. Mycoviruses are common among different species of endophytic fungi of grasses. Arch Virol 2009;154:327–30.
Rosseto P, Costa AT, Polonio JC, da Silva AA, Pamphile JA, Azevedo JL. Investigation of mycoviruses in endophytic and phytopathogenic strains of Colletotrichum from different hosts. Genet Mol Res 2016;15:15017651.
Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 2007;315:513–5.
Wang ZH, Zhao ZX, Hong N, Ni DJ, Cai L, Xu WX, et al. Characterization of causal agents of a novel disease inducing brown-black spots on tender tea leaves in China. Plant Dis 2017;101:1802–11.
McDougal R, Stewart A, Bradshaw R. Transformation of cyclaneusma minus with green fluorescent protein (gfp) to enable screening of fungi for biocontrol activity. Forests 2012;3:83–94.
Jia H, Dong K, Zhou L, Wang G, Hong N, Jiang D, et al. A dsRNA virus with filamentous viral particles. Nat Commun 2017;8:168.
Liu H, Fu Y, Jiang D, Li G, Xie J, Peng Y, et al. A novel mycovirus that is related to the human pathogen hepatitis E virus and rubi-like viruses. J Virol 2009;83:1981–91.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725–9.
Wang L, Jiang J, Wang Y, Hong N, Zhang F, Xu W, et al. Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: association with a coinfecting chrysovirus and a partitivirus. J Virol 2014;88:7517–27.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9:671–5.
Wu M, Jin F, Zhang J, Yang L, Jiang D, Li G. Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J Virol 2012;86:6605–19.
Zhang L, Fu Y, Xie J, Jiang D, Li G, Yi X. A novel virus that infecting hypovirulent strain XG36-1 of plant fungal pathogen Sclerotinia sclerotiorum. Virol J 2009;6:96.
Yoo S-D, Cho Y-H, Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2007;2:1565–72.
Doehlemann G, van der Linde K, Aßmann D, Schwammbach D, Hof A, Mohanty A, et al. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog 2009;5:e1000290.
Zhong J, Pang XD, Zhu HJ, Gao BD, Huang WK, Zhou Q. Molecular characterization of a trisegmented mycovirus from the plant pathogenic fungus Colletotrichum gloeosporioides. Viruses 2016;8:268.
Covelli L, Coutts RHA, Di Serio F, Citir A, Acikgoz S, Hernández C, et al. Cherry chlorotic rusty spot and Amasya cherry diseases are associated with a complex pattern of mycoviral-like double-stranded RNAs. I. Characterization of a new species in the genus Chrysovirus. J Gen Virol 2004;85:3389–97.
Jiang D, Ghabrial SA. Molecular characterization of Penicillium chrysogenum virus: reconsideration of the taxonomy of the genus Chrysovirus. J Gen Virol 2004;85:2111–21.
Ghabrial S, Castón A, Coutts JR, Hillman RHA, Jiang BI, Kim D. DH, et al. ICTV virus taxonomy profile: Chrysoviridae. J Gen Virol. 2018;99:19–20.
Lin YH, Hisano S, Yaegashi H, Kanematsu S, Suzuki N. A second quadrivirus strain from the phytopathogenic filamentous fungus Rosellinia necatrix. Arch Virol 2013;158:1093–8.
Kanhayuwa L, Kotta-Loizou I, Özkan S, Gunning AP, Coutts RHA. A novel mycovirus from Aspergillus fumigatus contains four unique dsRNAs as its genome and is infectious as dsRNA. Proc Natl Acad Sci USA. 2015;112:9100–5.
Ejmal MA, Holland DJ, MacDiarmid RM, Pearson MN. The effect of Aspergillus thermomutatus chrysovirus 1 on the biology of three aspergillus species. Viruses 2018;10:539.
Carroll G. Forest endophytes: pattern and process. Can J Bot 2011;73:316–24.
Wheeler DL, Dung JKS, Johnson DA. From pathogen to endophyte: an endophytic population of Verticillium dahliae evolved from a sympatric pathogenic population. N Phytol 2019;222:497–510.
Redman R, Freeman S, Clifton DR, Morrel J, Brown G, Rodriguez RJ. Biochemical analysis of plant protection afforded by a non-pathogenic endophytic mutant of Colletotrichum magna. Plant Physiol 1999;119:795–804.
Schulz B, Boyle C. The endophytic continuum. Mycol Res 2005;109:661–86.
Redman R, Dunigan D, Rodriquez R. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol 2001;151:705–16.
Álvarez-Loayza P, White J, Torrez M, Balslev H, Kristiansen T, Svennin J, et al. Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PLoS ONE 2011;6:e16386.
Hiruma K, Gerlach N, Sacrista S, Nakano RT, Hacquard S, Kracher B, et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell 2016;165:464–74.
Rai M, Agarkar G. Plant–fungal interactions: what triggers the fungi to switch among lifestyles? Crit Rev Microbiol 2016;42:428–38.
Freeman S, Rodriguez RJ. Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 1993;260:75–8.
Shah UA, Kotta-Loizou I, Fitt BDL, Coutts RHA. Identification, molecular characterization, and biology of a novel quadrivirus infecting the phytopathogenic fungus Leptosphaeria biglobosa. Viruses 2019;11:9.
Shah UA, Kotta-Loizou I, Fitt BDL, Coutts RHA. Mycovirus-Induced hypervirulence of Leptosphaeria biglobosa enhances systemic acquired resistance to Leptosphaeria maculans in Brassica napus. Mol Plant Microbe Interact. 2020;3:98–107.
Zhang H, Xie J, Fu Y, Cheng J, Qu Z, Zhao Z, et al. A 2-kb mycovirus converts a pathogenic fungus into a beneficial endophyte for brassica protection and yield enhancement. Mol Plant 2020;13:1420–33.
Xie J, Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol 2014;52:45–68.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31201488) and the Fundamental Research Funds for Environment and Plant Protection Institute, CATAS (No. 1630042019017). The authors would like to thank Jiatao Xie, Heng Kang, and Chaoxi Luo, College of Plant Science and Technology, Huazhong Agricultural University, Wuhan, China, for kindly assisting with the cloning experiments, endophyte studies, and generously contributing the GFP plasmid, respectively. We also thank Robert H. A. Coutts for assistance with the English language and helpful discussions.
Author information
Authors and Affiliations
Contributions
W.X. designed the investigation and wrote the manuscript, L.Z. conducted experiments, including cloning, TEM, transfection, and pathogenicity assays, X.L. conducted experiments, including confocal laser scanning microscopy and biological analysis, S.L., D.N., N.H., and G.W. were involved in project supervision and funding acquisition, and I.K.-L. performed the statistical analysis and improved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, L., Li, X., Kotta-Loizou, I. et al. A mycovirus modulates the endophytic and pathogenic traits of a plant associated fungus. ISME J 15, 1893–1906 (2021). https://doi.org/10.1038/s41396-021-00892-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41396-021-00892-3
This article is cited by
-
Viruses of plant-pathogenic fungi: a promising biocontrol strategy for Sclerotinia sclerotiorum
Archives of Microbiology (2024)
-
A virus from Aspergillus cibarius with features of alpha- and betachrysoviruses
Virus Genes (2024)
-
Co-infection of Aspergillus ochraceopetaliformis strain RCEF7483 by a novel chrysovirus and a known partitivirus
Archives of Microbiology (2024)
-
Molecular characterization of a novel narnavirus infecting the phytopathogenic fungus Botryosphaeria dothidea
Archives of Virology (2024)
-
Molecular characterization of a novel penoulivirus from the phytopathogenic fungus Colletotrichum camelliae
Archives of Virology (2022)