Abstract
Lichens, symbiotic consortia of lichen-forming fungi and their photosynthetic partners have long had an extremely poor fossil record. However, recently over 150 new lichens were identified from European Paleogene amber and here we analyse crustose lichens from the new material. Three fossil lichens belong to the extant genus Ochrolechia (Ochrolechiaceae, Lecanoromycetes) and one fossil has conidiomata similar to those produced by modern fungi of the order Arthoniales (Arthoniomycetes). Intriguingly, two fossil Ochrolechia specimens host lichenicolous fungi of the genus Lichenostigma (Lichenostigmatales, Arthoniomycetes). This confirms that both Ochrolechia and Lichenostigma already diversified in the Paleogene and demonstrates that also the specific association between the fungi had evolved by then. The new fossils provide a minimum age constraint for both genera at 34 million years (uppermost Eocene).
Similar content being viewed by others
Introduction
Lichens are highly specialized mutualistic symbioses, in which a dominant fungal symbiont (mycobiont) hosts one or several taxa of phototrophic green algae and/or cyanobacteria (photobionts). The vast majority of the over 19 500 currently known species of lichen-forming fungi belong to the Ascomycota1,2. Many of them grow as tightly adhered crusts on their substrate, mostly on rock, soil, or bark. Crustose lichens are found in almost all major terrestrial biomes ranging from the tropics to polar regions.
In addition to the myco- and photobionts, lichen thalli commonly support diverse assemblages of associated microfungi and bacteria3,4,5. Lichenicolous fungi are a diverse group of obligate associates of lichens. A vast majority of them are ascomycetes, but the group also includes taxa from several classes of the basidiomycetes6,7. The specificity of different lichenicolous associations varies from general to highly specific6,8. Highly specific associations often involve specialized infection structures and exhibit relatively low virulence6.
Succinite is the major variety of amber (fossil resin) from large Paleogene European deposits located in the Baltic area (Baltic states, Poland, western Russia and adjacent regions) and near the city of Bitterfeld in central Germany. Succinite was recently shown to preserve numerous relatively well-preserved lichen fossils9, multiplying the known fossil record of lichens over tenfold. Regardless of the sometimes exceptional preservation of the amber inclusions and the utilization of modern research methods, a reliable identification of even the larger foliose and fruticose lichen inclusions is very challenging10,11. The anatomy of fossils preserved in amber can only rarely be studied, and information on many crucial characters such as spore size and septation, ascoma structure, and cortex type are rarely available. In many extant lichen lineages, lichen secondary chemistry provides important clues for distinguishing between taxa12 that cannot be examined from amber inclusions. Due to these limitations, only four extant lichen genera have unambiguously been identified from amber specimens so far, namely Anzia Stizenb. (Parmeliaceae), Calicium Pers. (Caliciaceae), and Chaenotheca (Th. Fr.) Th. Fr. (Coniocybaceae) from Paleogene European amber13,14,15,16, and Phyllopsora Müll. Arg. (Ramalinaceae) from Miocene Dominican amber17,18. Confidently assigned fossils provide minimum ages for the respective lineages and represent the standard for the calibration of divergence time estimations19. The still few confidently assigned lichen fossils have significantly deepened our understanding in the origins and evolution of the various lineages of Ascomycota15,20,21,‒22. Fossil evidence of interactions between microfungi and lichens have so far been limited to more general and likely saprotrophic associations of filamentous microfungi and decomposing lichen thalli23,24.
Most crustose lichens are relatively small and detaching them from the substrate is often impossible without major damage to the thallus. This has hindered the preservation and detection of crustose lichens in amber in two ways: firstly, large pieces of lichen substrate are rarely preserved, and secondly, any preserved fossils go easily undetected. For these reasons, with the exception of calicioid lichens, crustose species were until very recently absent from the fossil record. However, our recent survey demonstrated that crustose lichens are indeed present in European Paleogene amber9. In this study we analyse the most spectacular examples of fossil crustose lichens and also elucidate their associations with lichenicolous fungi.
Results
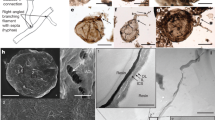
All studied fossils are fertile and represent lichen-forming species of the Ascomycota, preserved on degraded bark remains inside amber. Three fossils belong to the genus Ochrolechia A. Massal. (Ochrolechiaceae, Pertusariales; Figs 1a,b and 2a,b) and one is assigned to the order Arthoniales (Fig. 2h). Additionally, conidiomata and/or ascomata of the lichenicolous fungi Lichenostigma Hafellner (Lichenostigmatales; Fig. 1c–e) are preserved on two Ochrolechia specimens. Nine additional fossils represent crustose lichens which cannot with confidence be assigned to extant genera. For the detailed description of each fossil, see Supplementary material.
Fossil Ochrolechia specimens with lichenicolous Lichenostigma from European Paleogene amber. (a) Ochrolechia with apothecia, growing together with a foliose lichen (GZG.BST.21924). The black dots on the apothecial margin (c,d) are conidiomata and/or ascomata of the lichenicolous fungi Lichenostigma. (b) Ochrolechia with apothecia and (e) Lichenostigma on the crustose thallus (GZG.BST.27293). Scale bars 500 µm in (a,b), 100 µm in (c,e), 20 µm in (d).
Crustose lichens from European Paleogene amber. Ochrolechia (a,b), and unidentified crustose lichens (c,d). (a,b) Ochrolechia with apothecia, growing together with foliose lichens. (c) Apothecium. (d) Ascospores on the upper surface of the apothecial disk. (e) Unicellular ascospore. (f,g) Apothecia. (h) Specimen of Arthoniales with byssoid thallus and conidiomata. The optical sections of immersed conidiomata are seen from the lower side and show hyphae and masses of conidia preserved within the conidiomata. (a) GZG.BST.21924, (b) GZG.BST.27298, (c–e) GZG.BST.21982, (f) GZG.BST.21981, (g) GZG.BST.21915, and (h) GZG.BST.21925. Scale bars 1 mm in (a,b), 200 µm in (c,f–h), 50 µm in (d), and 10 µm in (e).
Systematic Paleontology
Division Ascomycota Cavalier-Smith, 1998
Subdivision Pezizomycotina O.E. Erikss. & Winka, 1997
Class Lecanoromycetes O.E. Erikss. & Winka, 1997
Subclass Ostropomycetidae Reeb, Lutzoni & Cl. Roux, 2004
Order Pertusariales M. Choisy ex D. Hawksw. & O.E. Erikss.
Family Ochrolechiaceae R.C. Harris ex Lumbsch & I. Schmitt, 2006
Genus Ochrolechia A. Massal., 1852
Description: Crustose lichens with apothecia (Figs 1a,b and 2a,b). Crustose thallus thin, vegetative diaspores not present. Apothecia sessile, large (0.9–2.0 mm in diameter), with prominent, smooth and even margins. Apothecial discs even or concave, pruina not visible.
Specimens examined: Collections of the Geoscience Centre at the University of Göttingen GZG.BST.21924, GZG.BST.27293, and GZG.BST.27298.
Remarks: Conidiomata and/or ascomata of the lichenicolous fungus Lichenostigma are present on the apothecial margin and crustose thallus of two specimens (Fig. 1c–e).
Class Lecanoromycetes O.E. Erikss. & Winka, 1997
Subclass, family & genus incertae sedis
Description: Corticolous crustose lichens with apothecia (Fig. 2c,f). Crustose thallus not visible. Apothecia sessile, irregular and angular in shape, 0.7–0.9 mm in diameter, with prominent, smooth to crenate and uneven margins. Apothecial discs even and possibly covered by thin pruina. Ascospores simple, elliptical, brown, and 15 × 9 µm in size (Fig. 2d,e; based on specimen GZG.BST.21982).
Specimens examined: Collections of the Geoscience Centre at the University of Göttingen GZG.BST.21981 and GZG.BST.21982.
Class Lecanoromycetes O.E. Erikss. & Winka, 1997
Subclass, order, family & genus incertae sedis
Description: Corticolous crustose lichens with apothecia (an example in Fig. 2g). Crustose thallus thin, degraded, or not visible. Vegetative diaspores rarely present (possible soredia present in one specimen). Apothecia sessile or rarely partially immersed in bark, round to oval, 0.1–0.7 mm in diameter. Apothecial margins (when observable) prominent to existent, often smooth and even. Apothecial discs (when observable) even and possibly covered with thin pruina.
Specimens examined: Collections of the Geoscience Centre at the University of Göttingen GZG.BST.21915, GZG.BST.21930, GZG.BST.21941, GZG.BST.21942, GZG.BST.21983, GZG.BST.21984, and GZG.BST.21985.
Remarks: The specimens may represent several taxa. However, the preservation does not allow their assignment to extant genera. On the crustose thallus of two specimens, cell-chains and/or conidiomata/ascomata of possibly lichenicolous fungi are present (Fig. S1).
Class Arthoniomycetes O.E. Erikss. & Winka, 1997
Order Arthoniales Henssen ex D. Hawksw. & O.E. Erikss., 1986
Family and genus incertae sedis
Description: Corticolous crustose lichen with a byssoid thallus and numerous conidiomata (Fig. 2h). Conidiomata dark, slightly oval and ca. 0.2 mm in diameter, filled with light masses of conidia.
Specimens examined: Collections of the Geoscience Centre at the University of Göttingen GZG.BST.21925
Class Arthoniomycetes O.E. Erikss. & Winka, 1997
Order Lichenostigmatales Ertz, Diederich & Lawrey, 2014
Family Phaeococcomycetaceae McGinnis & Schell
Genus Lichenostigma Hafellner, 1983
Description: Lichenicolous fungi growing on crustose lichen thalli and apothecial margin (Fig. 1c–e). Conidiomata and/or ascomata up to 40 µm in diameter, consisting of subglobular cells of 2–5 µm size.
Specimens examined: Collections of the Geoscience Centre at the University of Göttingen GZG.BST.21924 and GZG.BST.27293.
Remarks: Lichenostigma is found growing on two Ochrolechia specimens. Conidiomata and ascomata of Lichenostigma are morphologically indistinguishable.
Discussion
Several studies on Ascomycota have recently utilized fossil fungi for the calibration of evolutionary analyses15,20,22. However, among the lichen-forming genera only the Paleogene Anzia (Parmeliaceae, Lecanoromycetes), Calicium (Caliciaceae, Lecanoromycetes), and Chaenotheca (Coniocybaceae, Coniocybomycetes) and Miocene Phyllopsora (Ramalinaceae, Lecanoromycetes) have been available to add confident minimum age constraints for lichenized and lichen-associated fungal genera. Identification of crustose lichens is very challenging even with extant specimens, and often without precise information on anatomy (e.g. ascus structure, apothecial margin anatomy or size and septation of ascospores) and the secondary chemistry (acetone-soluble lichen metabolites or insoluble pigments)25,26 even assignment to higher taxonomic categories is impossible. In this study we present fossils that can be used as new genus-level calibration points for Ochrolechia and Lichenostigma within the Pertusariales and the Lichenostigmatales by setting the minimum age of both genera to 34 million years.
Ochrolechia is a genus of mostly corticolous or saxicolous crustose lichens with relatively large and conspicuous apothecia with prominent margins. Of the studied fossil amber inclusions, several well-preserved specimens were identified as Ochrolechia based on general habit and a combination of morphological characters. The large, round to slightly oval apothecia with thick and prominent margins, together with the shape of the disc and the attachment of the apothecial exciple to the apothecial disc place the fossil specimens within Ochrolechia. Close morphological equivalents exist among extant Ochrolechia species: for example, specimen GZG.BST.21924 has large apothecia 1‒2 mm in diameter with prominent and even margins, and smooth discs (Figs 1a and 2a), very similar to O. subplicans (Nyl.) Brodo and O. xanthostoma (Sommerf.) K. Schmitz & Lumbsch while specimen GZG.BST.27293 has sessile, slightly oval, approximately 1.3 mm in diameter apothecia with very prominent, smooth, and even margins, and clearly concave discs (Fig. 1b), closely resembling O. balcanica Verseghy27,28.
In addition to DNA characters, the identification of the approximately 60 extant Ochrolechia species relies largely on secondary chemistry, but also the shape, size, and margin type of the apothecia and the presence of pruina are important for species determination28,29,30,31,32,33,34. In some extreme cases, only the geographical distribution, differences in the specific placement of lichen substances within thalli, or other subtle differences in lichen chemistry distinguish between morphologically cryptic, but genetically distinct species34,35. Because of obvious limitations in the biochemical analysis of amber-preserved lichens, we cannot determine if the fossil Ochrolechia specimens represent one or several species.
The distinctly smaller size of the apothecia of the well-preserved fossils within amber specimen GZG.BST.21915 (Fig. 2g) and GZG.BST.21941 distinguish them from the Ochrolechia specimens. Additionally, the apothecial margins and general morphology of the apothecia resembles those of for example some extant Lecanora Ach. species. Amber specimens GZG.BST.21981 (Fig. 2f) and GZG.BST.21982 (Fig. 2c–e) contain well-preserved inclusions, and in the latter amber has even captured the ascospores that are in the process of being released from the apothecium (Fig. 2d). However, despite these interesting features, the fossils cannot be assigned to any one genus.
The fossil crustose lichen with a byssoid thallus and dark conidiomata resembles some extant taxa of the Arthoniales. This order includes five to six families of crustose and fruticose lichens36. The conidiomata of the fossil closely resemble those of some Inoderma and Lecanactis species37,38. However, the fossil cannot be assigned to any one genus, especially as it has recently been shown that the traditional morphology-based circumscription of taxa within Arthoniales does not correspond with gene-derived phylogenies36,39,40,41. The unusually high level of homoplasy indicate that Arthoniales is an ancient group of fungi and that the immediate precursors of some genera may have already existed in the Mesozoic36,42.
The genus Lichenostigma currently contains five lichenicolous species8,43, of which L. alpinum (R. Sant., Alstrup & D. Hawksw.) Ertz & Diederich is known from several extant species of Ochrolechia43. The conidiomata and/or ascomata of the extant Lichenostigma species are similarly sized and composed of identical subspherical cells as the fossil Lichenostigma. In addition to the Ochrolechia specimens, microfungi are present also in two other crustose lichen inclusions. The appearance of the fungi is similar to Lichenostigma but in addition to the conidiomata/ascomata, also moniliform hyphae is present (Fig. S1). Among the extant Lichenostigma, presence of mycelium is extremely rare, and, especially since we are lacking more detailed information about the host taxa of these fungi, it is possible that they rather represent some other taxa for example within Lichenostigma s. lat.43.
Previous studies have not been successful in estimating the divergence time of the family Ochrolechiaceae with any precision, with estimates spanning from the Late Cretaceous to the Paleogene20, and no previous divergence time estimates are available for Lichenostigmataceae. Our new findings show that both Ochrolechia and Lichenostigma were already present 34 million years ago, i.e., in uppermost Eocene for which Baltic amber provides a preservation window. In contrast to all lichen-associated filamentous microfungi previously described from European Paleogene amber23,24, Lichenostigma represents a true mycoparasite, providing the first fossil evidence of these highly specialized and ecologically important associations. It also demonstrates that the intimate link between Lichenostigma and its hosts is ancient and most probably traces back to the Mesozoic.
Materials and Methods
The studied fungal fossils (Table 1) are part of the collections of the Geoscience Centre at the University of Göttingen (GZG). Seven of the fossils are preserved in Baltic amber and six in Bitterfeld amber. Detailed descriptions of each specimen are included in the Supplementary material.
The Eocene sediments containing Baltic amber are 34–47 million years old44,45. Baltic amber primarily derives from the marine Blue Earth layer that is mainly mined on the Samland Peninsula northwest of Kaliningrad (Russia). Baltic amber eroded from sediments is abundantly found washed ashore along the coast of the Baltic Sea45,46. The absolute age of Baltic amber is still under debate. Palynological data suggest an upper Eocene (Priabonian) age (ca. 38–34 Ma) of the Blue Earth but fewer amounts of Baltic amber also occur in Lutetian (middle Eocene) sediments44,45,47.
Bitterfeld amber originates from the Goitzsche mine near the city of Bitterfeld in central Germany. It occurs in the Chattian ‘Bernsteinschluff’ Horizon in the upper part of the Cottbus Formation. The Upper Oligocene amber-bearing sediment has an absolute age of 23.8–25.3 million years48,49. A notion that Bitterfeld amber represents redeposited Eocene Baltic amber is based on the fact that there is a significant proportion of identical arthropod morphologies in amber from both localities50. However, redeposition of Baltic amber is unlikely, based on the reconstruction of the sedimentary environment of this large amber deposit45. A local reworking of pre-Chattian amber, however, has not been dispelled so far51. In any case, Bitterfeld amber is Paleogene in age and its minimum age is ca. 24 Ma.
The amber pieces were ground and polished manually using a series of wet silicon carbide papers (grit from FEPA P 600–4000, Struers, Germany) to produce smooth amber surfaces as close to the fossil inclusions as possible to minimize light distortion for imaging but still ensuring the preservation of the fossil. The fossils were examined under a Carl Zeiss Stereo Discovery V8 dissecting microscope and a Carl Zeiss AxioScope A1 compound microscope, each equipped with a Canon EOS 5D digital camera. In most cases, incident and transmitted light were used simultaneously. The images are digitally stacked photomicrographic composites obtained from up to 130 focal planes using the software package Helicon Focus (Version 6.3.3 Pro).
Data Availability
All specimens are part of the public collection of the Geoscience Centre at the University of Göttingen.
References
Feuerer, T. & Hawksworth, D. L. Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan’s floristic regions. Biodivers. Conserv. 16, 85–98 (2007).
Lücking, R., Hodkinson, B. P. & Leavitt, S. D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota – Approaching one thousand genera. Bryologist 119, 361–416 (2017).
Girlanda, M., Isocrono, D., Bianco, C. & Luppimosca, A. M. Two foliose lichens as microfungal ecological niches. Mycologia 89, 531–536 (1997).
U’Ren, J. M., Lutzoni, F., Miadlikowska, J., Laetsch, A. D. & Arnold, A. E. Host- and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 99, 898–914 (2012).
Aschenbrenner, I. A., Cardinale, M., Berg, G. & Grube, M. Microbial cargo: do bacteria on symbiotic propagules reinforce the microbiome of lichens? Environ. Microbiol. 16, 3743–3752 (2014).
Lawrey, J. D. & Diederich, P. Lichenicolous Fungi: Interactions, Evolution, and Biodiversity. Bryologist 106, 80–120 (2003).
Diederich, P., Lawrey, J. D. & Ertz, D. The 2018 classification and checklist of lichenicolous fungi, with 2000 non‐lichenized, obligately lichenicolous taxa. Bryologist 121, 340–425 (2018).
Millanes, A. M., Truong, C., Westberg, M., Diederich, P. & Wedin, M. Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the Biatoropsis-Usnea system. Evolution 68, 1576–1593 (2014).
Kaasalainen, U., Schmidt, A. R. & Rikkinen, J. Diversity and ecological adaptations in Paleogene lichens. Nat. Plants 3, 17049, https://doi.org/10.1038/nplants.2017.49 (2017).
Hartl, C. et al. Lichen preservation in amber: morphology, ultrastructure, chemofossils, and taphonomic alteration. Fossil Rec. 18, 127–135 (2015).
Kaasalainen, U. et al. Alectorioid morphologies in Paleogene lichens: new evidence and re-evaluation of the fossil Alectoria succini Mägdefrau. PLoS ONE 10, e0129526, https://doi.org/10.1371/journal.pone.0129526 (2015).
Crespo, A. et al. Phylogenetic generic classification of parmelioid lichens (Parmeliaceae, Ascomycota) based on molecular, morphological and chemical evidence. Taxon 59, 1735–1753 (2010).
Rikkinen, J. & Poinar, G. O. Jr. Fossilised Anzia (Lecanorales, lichenforming Ascomycota) from European Tertiary amber. Mycol. Res. 106, 984–990 (2002).
Rikkinen, J. Calicioid lichens from European Tertiary amber. Mycologia 95, 1032–1036 (2003).
Beimforde, C. et al. Estimating the Phanerozoic history of the Ascomycota lineages: combining fossil and molecular data. Mol. Phylogenet. Evol. 78, 386–398 (2014).
Rikkinen, J. et al. Calicioid lichens and fungi in amber – Tracing extant lineages back to the Paleogene. Geobios 51, 469–479 (2018).
Rikkinen, J. & Poinar, G. O. Jr. A new species of Phyllopsora (Lecanorales, lichen-forming Ascomycota) from Dominican amber, with remarks on the fossil history of lichens. J. Exp. Bot. 59, 1007–1011 (2008).
Kaasalainen, U. et al. A Caribbean epiphyte community preserved in Miocene Dominican amber. Earth Environ. Sci. Trans. R. Soc. Edinb. 107, 321–331 (2017).
Parham, J. F. et al. Best Practices for Justifying Fossil Calibrations. Syst. Biol. 61, 346–359 (2012).
Prieto, M. & Wedin, M. Dating the diversification of the major lineages of Ascomycota (Fungi). PLoS ONE 8, e65576, https://doi.org/10.1371/journal.pone.0065576 (2013).
Divakar, P. K. et al. Evolution of complex symbiotic relationships in a morphologically derived family of lichen-forming fungi. New Phytol. 208, 1217–1226 (2015).
Divakar, P. K. et al. Using a temporal phylogenetic method to harmonize family and genus-level classification in the largest clade of lichen-forming fungi. Fungal Divers. 84, 101–117 (2017).
Kettunen, E., Schmidt, A. R., Diederich, P., Grabenhorst, H. & Rikkinen, J. Lichen-associated fungi from Paleogene amber. New Phytol. 209, 896–898 (2016).
Kettunen, E., Schmidt, A. R., Diederich, P., Grabenhorst, H. & Rikkinen, J. Diversity of lichen-associated filamentous fungi preserved in European Paleogene amber. Earth Environ. Sci. Trans. R. Soc. Edinb. 107, 311–320 (2018).
Meyer, B. & Printzen, C. Proposal for a standardized nomenclature and characterization of insoluble lichen pigments. Lichenologist 32, 571–583 (2000).
Smith, C. W. et al. (eds) The lichens of Great Britain and Ireland (British Lichen Society, 2009).
Brodo, I. M. Studies of the lichen genus Ochrolechia. 1. A new classification for Pertusaria subplicans and P. rhodoleuca. Can. J. Bot. 66, 1264–1269 (1988).
Kukwa, M. The lichen genus Ochrolechia in Europe (Fundacja Rozwoju Uniwersytetu Gdańskiego, 2011).
Brodo, I. M. Studies in the lichen genus Ochrolechia. 2. Corticolous species of North America. Can. J. Bot. 69, 733–772 (1991).
Schmitt, I. & Lumbsch, H. T. Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol. Phylogenet. Evol. 33, 1–82 (2004).
Schmitt, I., Yamamoto, Y. & Lumbsch, H. T. Phylogeny of Pertusariales (Ascomycotina): resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. J. Hattori Bot. Lab. 100, 753–764 (2006).
Kukwa, M. Ochrolechia aegaea and O. alaskana, two species with gyrophoric and variolaric acids in the cortex. Graph. Scr. 21, 42–48 (2009).
Kukwa, M. The lichen genus Ochrolechia in Poland III with a key, and notes on some taxa. Herzogia 22, 43–66 (2009).
Ertz, D. et al. Ochrolechia kerguelensis sp. nov. from the Southern Hemisphere and O. antarctica reinstated from the synonymy of O. parella. Phytotaxa 280, 129–140 (2016).
Kukwa, M., Schmitt, I. & Ertz, D. Ochrolechia incarnata comb. nov. (Lecanoromycetes, Ascomycota), a distinct species of the O. parella group from Europe and Macaronesia. Phytotaxa 371, 119–126 (2018).
Ertz, D. & Tehler, A. The phylogeny of Arthoniales (Pezizomycotina) inferred from nucLSU and RPB2 sequences. Fungal Divers. 49, 47–71 (2011).
Frisch, A., Ohmura, Y., Ertz, D. & Thor, G. Inoderma and related genera in Arthoniaceae with elevated white pruinose pycnidia or sporodochia. Lichenologist 47, 233–256 (2015).
Ertz, D., Sanderson, N., Łubek, A. & Kukwa, M. Two new species of Arthoniaceae from old-growth European forests, Arthonia thoriana and Inoderma sorediatum, and a new genus for Schismatomma niveum. Lichenologist 50, 161–172 (2018).
Tehler, A. & Irestedt, M. Parallel evolution of lichen growth forms in the family Roccellaceae (Arthoniales, Ascomycota). Cladistics 23, 432–454 (2007).
Ertz, D. et al. Towards a new classification of the Arthoniales (Ascomycota) based on a three-gene phylogeny focussing on the genus Opegrapha. Mycol. Res. 113, 141–152 (2009).
Ertz, D., Bungartz, F., Diederich, P. & Tibell, L. Molecular and morphological data place Blarneya in Tylophoron (Arthoniaceae). Lichenologist 43, 345–356 (2011).
Tehler, A., Irestedt, M., Bungartz, F. & Wedin, M. Evolution and reproduction modes in the Roccella galapagoensis aggregate (Roccellaceae, Arthoniales). Taxon 58, 438–456 (2009).
Ertz, D., Lawrey, J. D., Common, R. S. & Diederich, P. Molecular data resolve a new order of Arthoniomycetes sister to the primarily lichenized Arthoniales and composed of black yeasts, lichenicolous and rock-inhabiting species. Fungal Divers. 66, 113–137 (2014).
Standke, G. Die Tertiärprofile der Samländischen Bernsteinküste bei Rauschen. Schriftenreihe für Geowissenschaften 7, 93–133 (1998).
Standke, G. Bitterfelder Bernstein gleich Baltischer Bernstein? – Eine geologische Raum-Zeit-Betrachtung und genetische Schlußfolgerungen. Exkurs.f. und Veröfftl. DGG 236, 11–33 (2008).
Weitschat, W. & Wichard, W. Baltic amber in Biodiversity of Fossils in Amber (ed. Penney, D.) 80–115 (Siri Scientific Press, 2010).
Kosmowska-Ceranowicz, B., Kohlmann-Adamska, A. & Grabowska, I. Erste Ergebnisse zur Lithologie und Palynologie der bernsteinführenden Sedimente im Tagebau Primorskoje. Metalla 66, 5–17 (1997).
Knuth, G., Koch, T., Rappsilber, I. & Volland, L. Concerning amber in the Bitterfeld region—geologic and genetic aspects. Hallesches Jahrbuch für Geowissenschaften 24, 35–46 (2002).
Blumenstengel, H. Zur Palynologie und Stratigraphie der Bitterfelder Bernsteinvorkommen (Tertiär). Exkurs.f. und Veröfftl. DGG 224, 17 (2004).
Weitschat, W. Bitterfelder Bernstein - ein eozäner Bernstein auf miozäner Lagerstätte. Metalla 66, 71–84 (1997).
Dunlop, J. Bitterfeld amber in Biodiversity of Fossils in Amber (ed. Penney, D.) 57–68 (Siri Scientific Press, 2010).
Acknowledgements
We thank Heinrich Grabenhorst (Wienhausen) and Christel and Hans Werner Hoffeins (Hamburg) who generously provided specimens for this study. The study was supported by the Alexander von Humboldt Foundation (grant to U.K.). We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the University of Göttingen.
Author information
Authors and Affiliations
Contributions
U.K. designed the study. U.K. and M.K. analysed the specimens. A.S. and U.K. imaged the specimens and prepared the figures. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaasalainen, U., Kukwa, M., Rikkinen, J. et al. Crustose lichens with lichenicolous fungi from Paleogene amber. Sci Rep 9, 10360 (2019). https://doi.org/10.1038/s41598-019-46692-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46692-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.