Abstract
Ethiopian honey wine is one of the country's most popular spontaneously fermented traditional alcoholic beverages. However, the final product of this natural fermentation system is frequently of poor and inconsistent quality. Furthermore, it makes the process difficult to predict, control, and correct. Thus, the main aim of this study was to develop a direct fermentation system for Ethiopian honey wine, Tej. After isolating fermentative microbial strains from Tej samples, they were subjected to intensive screening to fit to its purpose. Later, phenotypic and genotypic characterization, and inoculation of isolates to honey-must were performed sequentially. Finally, microbial interaction and physicochemical analysis, including volatile compounds profiling, were done for the inoculated samples. The identified isolates were strains of Saccharomycetaceae and Lactobacillaceae families. These strains showed a good ability to tolerate osmotic stress and a lower pH environment. Tej sample produced by mixed culture inoculation of Saccharomyces and Lactobacillus species showed similar physicochemical, volatile compounds, and sensory attributes values with that of the control sample. Thus, a mixture of Saccharomyces and Lactobacillus strains could be used as a starter culture to produce Ethiopian honey, Tej, without scarifying of its major quality attributes.
Similar content being viewed by others
Introduction
Traditional fermented foods and beverages are made from locally available raw materials using native microorganisms, indigenous knowledge and locally available utensils1. In Ethiopia, the production and consumption of traditional foods and beverages is a very common practice2. Ethiopian honey wine, Tej, is one of the popular traditional alcoholic beverages in the country3. It is typically a household commercial product sold for consumption at the point of production3,4. Like other traditional alcoholic beverages, Tej is produced by spontaneous fermentation of crude honey5,6. Due to the absence of direct inoculation, the fermentative microorganisms responsible for converting honey-water mixture to ethanol during Tej fermentation are thought to be found in raw materials and utensils2. This spontaneous honey fermentation could be the primary cause of inconsistency in the quality of Tej produced by different households7,8. The honey quality difference might also contribute to this variation in the physicochemical properties of the final product6.
Traditional Tej producers prefer crude honey instead of purified honey for the making a good quality Tej5. This crude honey is first mixed with water at a proportion of 1:3. Because of the increase in the price of honey, some producers substitute some portion of honey with cane sugar during Tej making4. The mixture is then left in mixing tank for an additional 3–4 days to complete its primary stage fermentation. It will then be filtered using clean cloth to remove sediment and suspended particles. Subsequently, leaves and stems of “gesho” (Rhamnus prinoides) are added to the previously fermented honey-water mixture. “gesho” (R. prinoides) is primarily added to mixture to enhance the flavor of final product4. Again, some Tej makers may also add barks from a selected tree and/or herbal ingredients to add a distinctive flavor to Tej5. The mixture is again allowed to ferment for an additional 8–21 days in anaerobic conditions to complete the final fermentation stage. After the mentioned fermentation period, it is now ready for consumption as a final product. Customers typically prefer Tej, which has a yellow color, a sweet taste, and a turbid appearance6.
So far, limited studies had been conducted towards to the development of direct inoculated Tej fermentation system. The very first full article on Tej by Bahiru et al.6 reported the physicochemical properties of Tej samples collected from Addis Ababa, Ethiopia. This report shows a considerable variation in some physicochemical properties for Tej samples collected from various households. After five years, the same authors reported the yeast and lactic acid flora of Tej by using phenotypic characterization method4. Similarly, Lactobacillus and Saccharomyces were the dominant taxa for the Tej samples collected from different part of the country7. However, the dominance level was substantially different within and across the samples collected from different households. Furthermore, as the fermentation period drew close to an end, the microbial dynamics moved to this microbial dominance9. Moreover, Tej exhibited good antioxidant activity, possibly inherited from honey and “gesho” (R. prinoides)7. The antioxidant capacity of Tej was also increased as the fermentation progressed to completion9. The ability of fermentative microorganisms to produce polyphenol and flavonoid through a complex microbial enzymatic system, as well as the continuous extraction of bioactive compounds from gesho as fermentation progressed to the end, are the most likely explanations for the increase in antioxidant activity during the Tej fermentation period9,10,11.
Previous reports on the physicochemical and microbial ecology of Tej did not provide enough information to modernize this spontaneous fermentation system4,6,7,8. The selection of starter culture strains and process variable optimizations are still open gaps to be filled for the complete transformation of this process. However, inoculated fermentation using single strain cultures might cause the fermented products to lose their peculiarity12. The main reason for this drawback is that the basis for selecting single strains is to minimize or maximize only one output function of the process13. Using a mixed culture is the best option for dealing with the drawbacks caused by both spontaneous and single strain inoculated fermentation systems14. Besides, using commercially available strains as a starter culture may result in substrate incompatibility, inability to adapt to stressful fermentation conditions, and/or loss of distinctive final product characteristics13,14. Thus, the purpose of this study was to isolate, screen, and characterize potential mixed starter cultures from Tej samples collected from various households, with the ultimate goal of developing an inoculated fermentation system for this traditional alcoholic beverage. Furthermore, we analyzed the physicochemical and volatile compounds to assess whether the isolated strain inoculations achieved the intended quality attributes of the final product.
Results
Isolation, characterization and screening of starter cultures
Based on macroscopic feature differences, 91 bacterial and 83 fungal colonies were isolated from Tej samples using de Man, Rogosa, and Sharpe (MRS) and yeast extract peptone dextrose (YPD) media. The catalase activity, gram reaction, and indole test to characterized the bacterial isolates. Indole negative, gram-negative, and catalase-negative characteristics were found in 67 of the 91 isolates. These bacterial and fungal isolates were subjected to further physiological tests. Isolates that could ferment carbohydrates and produce gas advanced to the next level of screening, which included the ability to grow at lower pH, varying temperatures, and higher sugar concentrations (Table 1). As a result, the number of isolates was reduced as the screening progressed to the end. A total of 34 presumptive heterofermentative Lactobacillus that could ferment carbohydrates and produce gas were screened from 67 bacterial isolates. Similarly, 49 fungal isolates that had a good ability to ferment carbohydrates were screened from the first isolated pool (Table 1). Subsequently, 27 and 31 bacterial and fungal isolates with a good ability to proliferate at lower pH (4.5) growth media were again screened. In this study, the isolates with an optical density (OD) value greater than 1.0 at 600 nm wavelength were considered as higher pH tolerant microbes. From the prior pool of isolates, 21 and 20 bacterial and yeast isolates were screened for their good ability to grow in extreme temperatures (Table 1). Finally, the ability of the isolates to grow at a higher sugar concentration was performed by using glucose concentrated MRS and YPD media. A total of 6 and 8 bacterial and yeast isolates with desirable characteristics were selected and subjected to genotypic identification and characterization.
Molecular identification of isolates
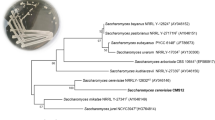
Genotypic identification of bacterial and yeast isolates was performed using 16SrRNA and ITS amplicon sequencing. The morphological features and genotypic identification of the isolates is presented in Table 2. In addition, the phylogenetic tree based on the 16S rRNA gene and ITS amplicon sequenced data of the isolates together with the closely related species is illustrated in Fig. 1a. All bacterial isolates had shown greater than 98.5% similarity with the National Center for Biotechnology Information (NCBI) nucleotide sequence database (Table 2). Moreover, all isolated bacteria were a family of Lactobacillaceae (Fig. 1a). Lactobacillus hilgardii, Lactobacillus paracasei, and Lactobacillus parabuchneri were the identified species from 6 bacterial isolates (Table 2). The source of these isolates was Tej samples collected from different locations (Table 2). For instance, L. parabuchneri strains were isolated from Tej samples collected from Debre Markos, Ethiopia. Similarly, the rest two of Lactobacillaceae isolates were identified as L. paracasei. These strains were isolated from Tej samples collected from Addis Ababa and Bahir Dar, Ethiopia (Table 2). Based on ITS sequence data, identified isolates were the species of Saccharomycetaceae (Fig. 1a). Saccharomyces cerevisiae, Pichia fermentans, and Wickerhamomyces anomalus were the identified species from the collected Tej samples (Table 2). Six out of eight isolates were identified as S. cerevisiae strains. The strains of this species were isolated from Tej samples collected from all source locations. The other identified isolate using ITS sequence was P. fermentans strain. This strain was isolated from Tej samples collected from Bahir Dar and Addis Ababa, Ethiopia. The remaining isolate, W. anomalus strain, was isolated from Tej samples collected from Debre Markos (Table 2). Furthermore, phylogenetic relationship and genetic diversity analysis were conducted for the isolated strains of the same species. Randomly amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) and Microsatellite multiplex PCR were used to achieve these objectives. All S. cerevisiae isolates were subjected to the above-mentioned analysis, with the exception of P. fermentans and W. anomalus, which were isolated only once. All strains of Lactobacillus species had clustered very narrowly together with very uniform DNA fragment pattern (Fig. 1b). Whereas, the strains of S. cerevisiae showed a higher level of polymorphism (Fig. 1b). Three strains of S. cerevisiae had shown a uniform DNA fragment pattern. The rest three S. cerevisiae strain isolates had shown a difference in the pattern of amplified DNA fragment on agarose gel electrophoresis. S. cerevisiae with the strain code AAF18 was chosen for further analysis and inoculation honey-must in this study based on the majority in similarity of presence. Since strains of the same species of Lactobacillus showed high level of genome similarity, we used a strain code DMB13, DMB33, and DMB23 to be used as mixed culture inoculum for honey-must fermentation.
Phenotypic properties of isolates
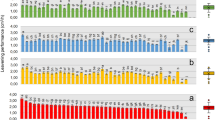
The characterization of isolates was further strengthened by phenotypic microarray (PM) analysis. PM 5, PM 9, and PM 10 microplates were used to achieve this purpose. The nutritional requirement of the isolates was determined using PM 5 microplate. PM 9 and PM 10 were utilized to evaluate the reaction of the isolates to osmotic stress and extreme pH environment. The isolated S. cerevisiae showed the best metabolism at the medium supplemented with D-biotin, nicotinamide, and (5)4-amino imidazole-4(5)-carboxamide (Fig. 2a). While the metabolism of P. fermentans isolate was best at the medium supplemented with inosine + thiamine, thiamine, and pyrophosphate. However, W. anomalus and almost all Lactobacillus isolates did not have any special preference over the nutrient supplements available on PM 5 (Fig. 2a). All Saccharomycetaceae isolates recorded a good metabolism at different concentrations of sodium nitrite, sodium lactate, sodium formate, sodium sulfate, ethylene glycol, and potassium chloride. Nevertheless, lower metabolism was recorded for these isolates in the growth medium containing high sodium chloride (NaCl) concentration (Fig. 2b). Similarly, all Lactobacillus isolates responded well to increased sodium nitrite, sodium sulfate, and ethylene glycol concentrations. Like Saccharomycetaceae isolates, all the Lactobacillus isolates had difficulties for proper metabolism at a higher NaCl concentration. (Fig. 2b). Furthermore, PM 10 characterization revealed that Saccharomycetaceae isolates were more adaptable to lower pH environments than Lactobacillus species. The isolates of Lactobacillus species responded well to medium having pH values greater than or equal to 4.5 (Fig. 2c). Compared to other Saccharomycetaceae isolates, P. fermentans responded relatively better to different pH environments. Although they are not the same as P. fermentans, S. cerevisiae and W. anomalus had also demonstrated acceptable tolerance to a different pH condition (Fig. 2c).
Heat map of the phenotypic microarray results for Saccharomycetaceae and Lactobacillaceae isolates using different (a) Nutrient supplements (b) Osmolytes (c) pH microplates. The magnitude of microbial metabolism is illustrated by a different heatmap color. The larger magnitude is indicated by dark red, while the magnitude gradually falls to light red, white, light blue, and finally dark blue, with the latter denoting a lesser magnitude.
Interaction between dominate species
Eight Tej samples were produced by inoculating isolated Lactobacillus and Saccharomycetaceae species in various combinations as a mixed starter culture. Other than pasteurization and inoculation with a defined starter culture, all the processes were conventional. The Tej samples from one to four were entirely inoculated by the isolates of Saccharomycetaceae. Other samples from five to eight were inoculated by the isolates of both Lactobacillus and Saccharomycetaceae strains. Tej that was spontaneously fermented and widely regarded as the best Ethiopian honey wine was used as a control sample. The details of the production process and the nomenclature of the samples are described in the “Methods” section. Plate count methods for total fermentative yeast and Lactobacillus were used to study the interaction of the inoculated microbes during the fermentation period. The total fermentative yeast growth rate for the TS1 sample had the highest exponential growth rate compared to other Tej samples inoculated with Saccharomycetaceae only (Fig. 3a). Besides, this sample reaches a stationary phase after 14 days of fermentation. Sample TS3 started at a higher cell count of 6 logs CFU/mL and reached the maximum (8 logs CFU/mL) after 14 days of fermentation. Sample TS2 and TS4 showed a gentle growth rate compared to TS1 and TS3. Especially the TS4 sample showed a slower growth rate than other yeast-only inoculated samples. However, this and TS2 samples showed an active growth rate even after 21 days of fermentation (Fig. 3a). Sample TS5, inoculated with a mixed culture of S. cerevisiae and L. hilgardii strains, grew rapidly in total fermentative yeast and Lactobacillus cell count. However, the growth rate of fermentative yeast was by far higher than that of Lactobacillus. The cell count of Lactobacillus was plateaued at 4 logs CFU/mL after 21 days of fermentation. In contrast, the cell count for total fermentative yeast reaches 7 logs CFU/mL at the end of fermentation (Fig. 3b). A similar higher fermentative yeast count was observed for the TS6 sample compared to its co-inoculated Lactobacillus count. After seven days of fermentation, the Lactobacillus cell count enters to stationary phase with a cell count of 3 logs CFU/mL. In contrast, the total fermentative yeast of this sample showed a higher growth rate and reached a maximum cell count of 7 logs CFU/mL at the end of its fermentation period (Fig. 3c). A proportional growth rate was observed for yeast and Lactobacillus during the TS7 Tej sample fermentation period. Nonetheless the growth rate of yeast was higher than the growth rate of Lactobacillus (Fig. 3d). At the end of fermentation, this sample recorded 5 and 4 logs CFU/mL count for total fermentative yeast and Lactobacillus cell counts, respectively. Sample TS8 started its fermentation with a higher Lactobacillus cell count (4 logs CFU/mL) and continued its dominance up to 14 days of fermentation. However, the cell count of fermentative yeast overtook this dominancy after 14 days. At the end of the fermentation period, the total fermentative yeast and Lactobacillus count reached 6 and 5 logs CFU/mL, respectively (Fig. 3e). The control sample also showed a similar microbial growth pattern to the previous mixed culture inoculated Tej samples. Specifically, the total fermentative yeast was a bit higher (4 logs CFU/mL) than the other inoculated test Tej samples. Nevertheless, the final cell concentration for both cell counts was similar to other test samples (Fig. 3f).
Microbial growth curve of (a) total fermentative yeast for TS1, TS2, TS3 and TS4 (b) total fermentative yeast and Lactobacillus for sample TS5 (c) total fermentative yeast and Lactobacillus for sample TS6, (d) total fermentative yeast and Lactobacillus for sample TS7 and (e) total fermentative yeast and Lactobacillus for sample TS8 (f) total fermentative yeast and Lactobacillus for the control sample.
Physicochemical characteristics
The physicochemical properties of Tej samples produced by inoculation of different mixed culture combinations are presented in Table 3. Significantly a higher (P < 0.05) glucose (14.85 g/L) level was observed for the TS1 sample. In contrast, the TS8 sample (9.85 g/L) and control sample (9.75 g/L) had significantly lower glucose levels (P < 0.05). Similarly, the fructose concentration of the TS2 sample was much higher, while the TS8 sample fructose level was significantly lower (P < 0.05). Generally, the control and mixed culture fermented samples showed lower sugar content than other inoculated samples (Table 3). Likewise, the ethanol level of the control sample had a significantly higher (P < 0.05) ethanol level (10.43 g/100 mL) than other test samples. In contrast, the TS2 sample had a significantly lower (P < 0.05) ethanol level of 7.87 g/100 mL than other inoculated samples (Table 3). Compared to samples inoculated with solely yeast culture, samples inoculated with a mixed culture of Lactobacillus and S. cerevisiae had a greater ethanol content (Table 3). Moreover, the lactic acid levels of fermented honey wines ranged from not detectable to 3.14 g/L. Except for sample TS8 with a very high lactic acid concentration, honey wines inoculated with a mixed culture of Lactobacillus and S. cerevisiae had roughly the same lactic acid concentration (Table 3). Similarly, the titratable acidity was lower for the samples inoculated with yeast combinations than samples inoculated with S. cerevisiae and Lactobacillus. Besides, the spontaneously fermented control sample had shown a higher titratable acidity (6.37 g/L).
Volatile compound and sensory attributes
Esters, alcohols, carbonyl compounds, alkanes, and acids were the major compounds identified and quantified from direct inoculated honey wines samples. From the aforementioned major volatile compounds, alcohols and esters were by far the most dominate volatile compounds in all of honey wines samples (Table 4). For instance, alcohols contributed subtotal values ranging from 728.9 mg/L (control sample) to 577.56 mg/L (TS3 sample). Particularly, ethanol was the most abundant volatile compound identified and quantified in all samples. The mean value of this compound ranged from 321.20 to 448.80 mg/L (Table 4). In this study, phenylethyl alcohol was the second most dominant volatile compound in all fermented honey wine samples. The value of Phenylethyl alcohol ranged from 219.79 to 274.50 mg/L (Table 4). About 13 ester compounds found in all samples, including the control, were identified and quantified. Especially, the ethyl ester of octanoic acid, dodecanoic acid, benzoic acid, and hexanoic acid was the common ester observed for all samples (Table 4). Besides, isoamyl acetate was the major compound for TS1, TS2, TS3, TS6, and control samples. In contrast, this compound was below the detectable limit for the TS7 and TS8 samples. The other volatile compound found in the majority of the samples was alkanes, with average mean values ranging from 13.14 mg/L (TS3 sample) to 43.29 mg/L (TS5 sample). For all fermented honey wine samples, tetradecane was the dominant volatile compound under the alkanes group (Table 4). Although cyclopentasiloxane, dodecamethyl- compound was not detectable in the TS3 sample, it was the most abundant compound in all samples compared to other compounds in the alkanes category (Table 4). Benzaldehyde, which was observed in all samples, was the major volatile compound in the carbonyl group. Furfural, observed in some of the samples, was another minor compound in this category. Volatile acids, which ranged from 16.64 to 29.87 mg/L, were the other significant volatile compounds found in the fermented honey wine sample (Table 4). The volatile phenol 2,4-Di-tert-butyl-phenol, Silanediol, dimethyl-, and Benzene the other significant volatile compound observed almost in all of the samples (Table 4). The volatile compounds that were not observed in the majority of the samples were then plotted on the canonical correspondence analysis (CCA) plot. The compounds such as Benzaldehyde, 3,4-dimethyl-, 1-Butanol, 3-methyl-, 2-Pentanol, Formate, Benzoic acid, Hexadecane were only observed in one or two of fermented honey wine samples (Fig. 4a). Furthermore, a Bray–Curtis principal coordinate analysis (PCoA) plot was utilized to assess dissimilarity between the honey wine tests and control samples based on the detected volatile components. Based on volatile compounds, TS5, TS8, and control samples clustered together on the PCoA plot, especially along the PCoA1 axis (Fig. 4b). Moreover, a subjective analysis of honey wine test samples was performed using a seven-point hedonic sensory score test. Color, turbidity, alcohol aroma, astringency, honey-like aroma, and sourness were the tested sensory attributes of the samples. The color of all test and control Tej samples had scored almost a similar result with no significant difference (P > 0.05) (Fig. 4c). Nevertheless, there was a significant difference for other sensory attributes between the test samples (P < 0.05). Furthermore, TS5, TS6, TS7, and control samples showed a cluster pattern for most sensory attributes (Fig. 4c).
The volatile compound and sensory attributes of honey wine samples inoculated with various strain combinations (a) Canonical correspondence analysis (CCA) plot of minor volatile compounds found in a small number of test samples (b) Principal coordinate analysis (PCoA) plot with Bray–Curtis dissimilarity for honey wine test samples based on volatile compound concentration (c) Radar plot for the sensory analysis results of the test samples using a seven-point hedonic scale.
Discussion
Ethiopian honey wine, Tej, is produced by a spontaneous fermentation process4,5. However, this kind of spontaneous fermentation has the main drawback on the predictability and controllability of the process12. To shift from spontaneous to direct fermentation, without losing any of its major quality attributes, studying the microbial ecology and development of starter culture is the major task1. The previous studies on Tej samples had clearly pointed out the microbial ecology was dominated by the species of Saccharomyces and Lactobacillus7,9,15. Thus, developing a starter culture composed of a mixture of Lactobacillus and Saccharomyces species will help achieve a direct Tej fermentation system without scarifying the wholesomeness of the final product.
The strains of Saccharomycetaceae (S. cerevisiae, W. anomalus, and P. fermentans) were isolated from Tej samples. These isolates were obtained after subjecting many isolates to intensive screening. Since they are the most responsible species for converting carbohydrates to ethanol, S. cerevisiae is the most expected strain in every alcoholic beverage16. Similar S. cerevisiae isolates were obtained from Tej and other Ethiopian traditional beverages4,17. Although it is uncommon to use W. anomalus and P. fermentans isolates as the sole starter culture for alcoholic beverage fermentation, co-culturing them with S. cerevisiae to improve the flavor of the alcoholic beverage is currently attracting the interest of many scientists18,19,20. Thus, the isolated Saccharomycetaceae strains have a good potential to be used as a starter culture for the fermentation of Ethiopian honey. However, W. anomalus and P. fermentans were not isolated from all sample collection areas. This could be due to the inherent microbial variability of the samples caused by the spontaneous nature of fermentation, or it could also be due to the stringent screening procedure4.
Lactobacillus species (L. hilgardii, L. paracasei, and L. parabuchneri) were the other strains isolated from Tej samples. Usually, lactic acid bacteria are introduced to wine after the completion of alcoholic fermentation or at the start of malolactic fermentation21. However, in Ethiopian honey wine, Lactobacillus and Saccharomyces are involved in the fermentation from the beginning up to the end of the fermentation period9. Thus, isolating Lactobacillus strains from Tej samples was not a surprising result. Especially, strains of L. hilgardii had been commonly involved in the alcoholic fermentations22. Besides, all of the isolates were under the heterofermentative Lactobacillus category. The Lactobacillus under this category has the ability to produce lactate, ethanol, and carbon dioxide from a given carbon source23. Furthermore, some of the Lactobacillus species enhance the flavor of the produced alcoholic beverage24. Similar lactic acid bacterial isolates for the Tej sample were obtained by applying phenotypic microbial characterization techniques4.
The Lactobacillus isolates clonal relationship were studied by using RAPD-PCR DNA fingerprinting. Because it amplifies fragments of genomic DNA, RAPD is one of the most powerful tools for studying genetic variation in living organisms25. However, there is still a loophole of this method on the reproducibility due to mismatch in annealing26. In this study, the isolated Lactobacillus strains of the same species did not show a high level of polymorphism. The primary justification that could be forwarded for this result could be due to intensive screening for harsh environment might cause isolated strains to have a similar allelic diversity. The luck of reproducibility, accuracy and application of limited number of primers during RAPD-PCR amplification could also be the other factor for this particular result25,26. The genetic diversity of S. cerevisiae strains was also studied by using Microsatellite multiplex PCR. Microsatellites, also known as simple sequence repeats (SSRs), are short repeats of DNA sequence motifs found in many genomes27. Among six S. cerevisiae strains three of them had shown high level of polymorphism. The inherent high genetic diversity of S. cerevisiae strains could be the possible major reason for these variations. However, the number of analyzed loci still had the influence on the genetic diversity of the strains28. Thus, it is worth to know that, the current result could have been different if more than three loci of the total genome had been amplified. One of the three S. cerevisiae strains (strain code AAF18) that showed similar allelic diversity was chosen for inoculation and phenotypic characterization in this study.
In addition to the aforementioned biochemical characterization of isolates, further characterization was performed using phenotypic microarray. The metabolism of S. cerevisiae and P. fermentans was high in the presence of vitamins (D-biotin, nicotinamide, thiamine), amides ((5)4-amino imidazole-4(5)-carboxamide), and phosphorous compound (pyrophosphate). The high metabolism could be due to the micronutrient (vitamins and minerals) requirements of microbe for the proper metabolism of cells29. Moreover, Saccharomycetaceae and Lactobacillus isolates had exhibited an excellent osmotic tolerance ability. This tolerance could be due to the adaptation of isolates to higher osmotic pressure experienced during honey wine fermentation. However, a lower magnitude of metabolism for all isolates was observed for the growth medium containing greater than 6% NaCl concentration. This result could be due to the cellular metabolic shift of isolated microbes from hyperplasia to hypertrophy at higher NaCl concentration30. Since honey-must has a lower pH by itself, and the pH continues to drop as fermentation progresses, tolerance to acidic stress is one of the essential characteristics of isolates to look for when choosing a starter culture. However, a lower pH can charge the biological molecules, which later can negatively impact both structure and function of cells31. In this study, Saccharomycetaceae responded well to lower pH environments than Lactobacillus isolates. This response could be due to the better membrane stability of fungi over bacteria. Especially for a pH lower than 4.5, the magnitude of metabolism for Lactobacillus isolates was very low. Pichia fermentans, conversely, had a strong tolerance for low pH than any other isolates. A similar Pichia isolates tolerance over the other Saccharomyces isolates was observed in the previous study32.
Following the extensive characterization of isolated microorganisms, it had been discovered that they had a strong chance of being employed as a starter culture for Tej fermentation. However, their growth in honey-based media and interaction with each other was the important concept to be covered for developing a starter culture. Fermentative yeast and Lactobacillus count were performed five times throughout the fermentation period of Tej. The availability of ample nutrients and little microbial competition causes a higher fermentative yeast count for the TS1 sample, especially at the early fermentation stage33. A similar increment of fermentative yeast for sample TS3 could be due to a higher initial microbial count of inoculum and higher availability of the required nutrient33,34. Surprisingly, the slower growth rate of fermentative yeast for sample TS4 could be caused by either mutual inhibition or the slow growth rate of one of the inoculums. The fast growth rate of Saccharomyces was observed compared to that of Lactobacillus in a mixed culture inoculated Tej fermentation. This fast growth rate could be caused either by the inhibition of Lactobacillus by Saccharomyces species or due to the inherent fast growth rate characteristics of Saccharomyces35,36. However, the growth rate of Lactobacillus itself followed almost a similar pattern as it was discussed in the previous study on Tej microbial dynamics9. Similar studies on the co-inoculation of Saccharomyces and Lactobacillus had revealed a production of good quality characteristics alcoholic beverage37. Moreover, the final product physicochemical and volatile compound analysis of Tej samples was performed to assess whether or not the inoculated fermentation had met the intended goal. In terms of residual sugar concentration, all test samples fell within the range observed in previous survey studies for Tej samples6,7,8,9. This result is essentially a positive testimony for the ability of the inoculum to utilize carbon sources from honey for cellular growth and the production of exo-metabolic products38. Similarly, test samples showed good ethanol concentrations, particularly for the product inoculated with a mixed culture of S. cerevisiae and Lactobacillus isolates; this could be due to the co-inoculation of heterofermentative Lactobacillus isolates, resulting in additional ethanol production39. Besides, the substantial lactic acid concentration was especially observed for the samples co-inoculated with S. cerevisiae and Lactobacillus isolates and the control sample. Moreover, TS3 and TS4 samples also showed the same lactic acid concentration, probably due to the lactic acid-producing yeast or due to lactic acid bacteria trace contamination. Similar lactic acid production by yeast was observed during the fermentation of beer and mead40,41.
The volatile compound analysis will help to objectively investigate the influence of different inoculum combinations on fermented Tej samples. The volatile compound of the honey wine is derived either from raw materials or from fermentative microorganisms42. Nevertheless, esters and alcohols were the most dominant volatile compounds in all test samples, including those of the test and control samples. Since honey wine is an alcoholic beverage, the presence of alcohol and alcohol derivative volatile compounds is expected to observe42,43. During alcoholic fermentation and slow aging, alcohol acetyltransferase and other enzymes could catalyze the formation of esters from an activated fatty-acyl CoA compound and alcohol44. Isoamyl acetate, the most abundant ester in Tej samples, is the compound responsible for fruity and floral notes and is typically derived from yeasts45. Moreover, Octanoic acid, ethyl ester, the second most abundant ester found in all samples, give pear, brandy, and lentil flavor to the produced Tej46. This volatile compound is usually the product of the fermentation process47. Esters, which contribute to the fruity aroma of wines, are generally good indicators of fermented beverages' young age48. Like honey wine samples treated with different fining agents49, alcohol was also the most dominant volatile compound in all of Tej samples. The dominant alcohols (ethanol and phenylethyl alcohol) were the compounds responsible for the vinous odor and pungent taste of alcoholic beverages50. Furthermore, higher alcohols (1-Propanol, 2-methyl-, 2,3-Butanediol, and 2-Heptanol) in the tested samples also contributes to the good flavor of honey wine49. These higher alcohol content and proportion played a significant role in improving the wine taste51. Besides esters and ethanol, other volatile compounds including carbonyl groups independently and interactively play a none dimensioning effect for the test and flavor of honey wine52. Furthermore, alkanes were also the other major group of volatile compounds observed in Tej samples. Cyclopentasiloxane compounds, in particular, were found in almost all of the samples. These silicon-based alkenes in the samples could be due to GC column leaching or bleeding. However, other studies on the volatile compounds of fermented beverages products have also found these cyclopentasiloxane base compounds in their test samples52,53. The volatile compound absent in the majority of the samples and with a lower concentration was plotted on the CCA plot. The majority of these compounds (3,4-dimethyl-, 1-Butanol, 3-methyl-, 2-Pentanol, Formate, Benzoic acid) plotted on CCA are volatile compounds derived from microbial fermentation process54. Furthermore, TS5, TS8, and control samples showed some kind of cluster on the Bray–Curtis dissimilarity PCoA plot. The clustered sample is a good indication of the similarity of the compounds for the samples mentioned above because the PCoA plot was generated from total volatile compounds55. The sensory analysis also revealed that these samples had a cluster pattern on the radar plot of a seven-point hedonic scale score for alcoholic aroma, astringency, sourness, and overall acceptance of sensory quality attributes. These sensory attributes are actually the results of volatile compounds present in the samples56. Specifically, these samples were made from honey-must fermentation inoculated with a mixed culture (Saccharomyces and Lactobacillus).
Conclusions
The isolated strains of Saccharomycetaceae and Lactobacillaceae for the purpose of starting honey wine fermentation had demonstrated good tolerance to osmotic pressure and a lower pH environment, as well as requiring minimal micro-nutrition These isolates, with different combinations, were applied to honey-must to assess its ability to produce a good quality Tej. Generally, test samples inoculated with a mixed culture of Saccharomyces and Lactobacillus strains had lower residual sugar content and higher ethanol level. Besides, the volatile compounds and sensory attributes for these samples were consistent with the control sample. Specifically, TS5 and TS8 samples, inoculated by different combinations of S. cerevisiae, L. hilgardii, L. parabuchneri, and L. paracasei, had a close quality attribute resemblance with that of the control sample. Thus, Ethiopian honey wine can be fermented using a direct inoculation system with either S. cerevisiae and L. hilgardii or S. cerevisiae and other Lactobacillus isolates (L. hilgardii, L. parabuchneri, and L. paracasei) without losing its major quality attributes.
Methods
Sample collection, transportation and storage
A total of 21 Tej samples containing presumptive starter culture strains were collected aseptically from three locations in Ethiopia (Addis Ababa, Bahir Dar and Debre Markos) using sterile screwed cap. These samples were obtained from alcohol retailers willing to sell their product for research purposes. All the Tej samples were then transported to the point of analysis via ice box containing freeze pack. Besides, samples which needs further analysis were stored in a refrigerator at temperature of 4 °C.
Enumeration and isolation of presumptive starter cultures
Yeast enumeration and isolation were carried out using YPD agar57. Chloramphenicol (100 μg/mL) was added to the growth media after autoclaving and cooling to 50 °C to inhibit bacterial growth. Each Tej sample (1 mL) was mixed with 9 mL of sterile saline solution (0.85% NaCl) to produce a serially diluted inoculating sample. After inoculation, the pet reddish was incubated at a temperature of 30 °C for a period of 48 h. At the same time, presumptive Lactobacillus enumeration and isolations were done via MRS agar medium and incubated under the anaerobic condition at 30 °C for 72 h. The macroscopic features (shape, size, pigment, surface, elevation, and opacity) of yeast and Lactobacillus colonies of different types were streaked on the same respective mediums. About 15 colonies of each representative type were re-streaked on the same medium to obtain pure colonies.
Phenotypic and physiological characterization
Presumptive Lactobacillus isolates were subjected to gram reaction, catalase activity, and indole tests according to methods described by Saarisalo et al. (2007)58. Then gram positive, catalase, and indole negative bacterial together with yeast isolates were subjected to purpose-oriented screening. The ability of isolates to ferment carbohydrate and to produce carbon dioxide (CO2) was conducted using Durham tubes according to standard protocol59. Then, both isolates (presumed yeast and Lactobacillus) with the ability to ferment glucose, and sucrose and produce CO2 were passed on to the next stage of the screening process. The ability to grow at high sugar concentrations was tested by applying 10%, 18%, and 25% glucose on growth media60. The microbial isolates with high sugar concentration tolerance were subjected to a third test, which was the ability to grow at lower pH levels of 3.5, 4.0, and 4.5. Both bacteria and yeast isolated with the ability to grow at a lower pH were subjected to a temperature sensitivity test. Thus, all isolates screened using the methods mentioned above were further tested for their ability to grow at temperatures of 15 °C, 25 °C, and 35 °C. Isolates that performed well in the above purpose-oriented screening were further subjected to genotypic characterization.
Genotypic identification and characterization
After extracting DNA from the isolated strains, ITS and 16sRNA amplicon sequencing were performed for the identification of yeast and Lactobacillus isolates, respectively. The identified Lactobacillus strains were then subjected to RAPD-PCR analysis by applying the methods used by Kostinek et al.61. Similarly, all S. cerevisiae isolates were subjected to microsatellite multiplex PCR analysis using the method developed by Vaudano and Garcia-Moruno62. Three microsatellite loci, SCPTSY7, SC8132X, and YOR267C were used for their high degree of polymorphism. Both RAPD-PCR and microsatellite multiplex PCR products were separated by using 1.8% (w/v) agarose gel electrophoresis system. After pattern processing and cluster analysis, a dendrogram was developed by using UPGMA method.
Phenotypic microarray analysis
PM assay for our isolates was performed by following manufacturer protocol of PM Technology (Biolog, Hayward, CA, USA). PM micro-panels are 96-well microplates with different substrates in each well. This study used PM5, PM9, and PM10 assays to determine nutrient requirements, osmotic/ionic stress responses, and sensitivity to different pH environments. Each well of the panels contains the required minimal medium components, specific dye, and unique substrate. The DNA amplicon sequenced bacterial and yeast isolates from Tej samples were chosen for this analysis. All test strains were first incubated overnight at a temperature of 30 °C on SMA (Standard Methods Agar, BioMérieux) medium. The cells were then collected from the SMA medium with a sterile cotton swab and dispersed in a sterile capped tube containing 20 mL of the inoculation fluid (IF-0, Biolog Inc.). Using a Biolog turbidimeter, the cell concentration was adjusted to 81% transmittance after all the bubbles created during cell dispersion had settled. After that, PM5, PM9, and PM10 plates were inoculated with the cell suspension (100 μL per well) and incubated at a temperature of 33 °C for about 120 h in the Omnilog Incubator/Reader (Biolog Inc., Hayward, USA). The color changes in the wells were measured once every 15 min, allowing for both amplification and quantification of the phenotype using OmniLog® data collection Software v 1.2 (Biolog Inc.).
Mixed culture fermentation
Starter culture preparations
Three Lactobacillus isolates were first inoculated into a 250 mL conical flask with MRS broth and incubated at 30 °C for 24 h under anaerobic conditions. Similarly, three yeast isolates were inoculated into a 250 ml Erlenmeyer flask with YPD broth and incubated at a temperature of 30 °C for 18 h. Following the incubation period, the growing mediums were centrifuged at 3500 rpm for 10 min to obtain a higher cell concentration. It was then washed twice using sterile water and resuspended again in saline solution (0.85% NaCl). Finally, the concentration of the suspended inoculums was adjusted to achieve the required final microbial concentrations.
Inoculated Tej fermentation
Tej was made in the lab by using inoculated fermentation and the same procedures as when it was made traditionally3,5,7,9. The same honey-must was used for all of the samples, which was made by mixing honey and water in a 1:3 ratio, and the concentrations of glucose and fructose were obtained 155 g/L and 210 g/L, respectively. Then, this mixture was pasteurized at 65 °C for 10 min. The pasteurized honey-water mixture was then cooled and aseptically transferred to a ten-piece set of 50-mL conical flasks with top screw caps. Taking the previous literatures7,8,9 and preliminary findings as a baseline, the prepared inoculums were mixed with different strain combinations at varying concentrations to create eight Tej samples. The first Tej sample (TS1) was prepared by inoculating S. cerevisiae (3 log cfu/mL) to honey-must mixture. The second sample (TS2) was inoculated with S. cerevisiae (3 log cfu/mL) and P. fermentans (2 log cfu/mL). The third sample (TS3) was inoculated with S. cerevisiae (3 log cfu/mL), P. fermentans (1 log cfu/mL), and W. anomalus (2 log cfu/mL). The fourth sample (TS4) was inoculated with S. cerevisiae (3 log cfu/mL) and W. anomalus (2.5 log cfu/mL). The fifth sample (TS5) was inoculated by S. cerevisiae (3 log cfu/mL) and L. hilgardii (2 log cfu/mL). The sixth test sample (TS6) was inoculated with S. cerevisiae (3 log cfu/mL) and L. parabuchneri (2 log cfu/mL). Similarly, sample seven (TS7) was inoculated with S. cerevisiae (3 log cfu/mL) and L. paracasei (2 log cfu/mL). The last experimental Tej sample (TS8) was prepared by inoculating a honey-water mixture with S. cerevisiae (3 log cfu/mL), L. hilgardii (2 log cfu/mL), L. parabuchneri (1 log cfu/mL)and L. paracasei (1 log cfu/mL). After inoculation, they were allowed to finish their primary fermentation stage for about 4 days at room temperature (26 °C). At this time, 1.5 g of boiled and sterilized gesho leaves and stems were added to the fermentation mediums (50 mL). The Tej-making process was completed after allowing these mixtures to spend for 20 more days.
Physicochemical analysis
Sugar analysis
The concentrations of glucose and fructose in the samples were determined using a High-Performance Liquid Chromatography-Evaporative Light Scattering Detector (HPLC-ELSD) system63. After centrifuging 5 mL Tej sample at 3200 rpm for 30 min, the supernatant was double filtered through 0.45 µm and 0.22 µm pore size membranes. Analyses were performed using an LC-20AT HPLC (Shimadzu, Japan), a YMC-Pack Polyamine II (250 4.6 mm. D.S-5 m, 12 nm) column, and an ELSD-LT II detector. At a column temperature of 30 °C, a 10 μL aliquot of the samples was injected into the HPLC-ELSD system. A mobile phase of acetonitrile: water at a 75:25 ratio with an isocratic pressure and a flow rate of 1.0 mL/min was used during the analysis period. Nitrogen (N2) was used as the nebulizer gas with a flow rate of 2 L/min. Finally, sugar quantification was performed by comparing the sugar peak areas of the samples to the corresponding standards calibration curves.
Ethanol quantification
The ethanol level was determined using the standard method developed by OIV (2020)64. A volumetric flask was first filled with a 200 mL aliquot of the Tej sample. The measuring cylinder was then rinsed three times with 20-mL distilled water. Additional distilled water (20 mL) was added to the distillate collection volumetric flask (200 mL). This collection flask was immersed in a cold-water bath throughout the distillation process. The distillate collection volumetric flask was then filled with deionized water to the markup. Finally, the alcohol content was determined using a standard aqueous organic solution table.
Lactic acid quantification
The lactic acid concentration in the Tej samples were quantified using spectrophotometric method developed by Borshchevskaya et al.65. A calibration curve was first constructed using serially diluted known concentration of DL-lactic acid stock solution (89%, ρ = 1.2 g/mL, Sigma-Aldrich). The absorbance of each serially diluted lactic acid stock (50 µL) solution was measured at 390 nm after it was mixed with 2 mL of a 0.2% solution of iron (III) chloride (FeCl3.6H2O). Similarly, the Tej samples were first centrifuged to remove the cellular particles from the fermented aliquot. The separated supernatant was then diluted 20-fold using deionized water. Subsequently, the test samples (50 µL) were mixed with 2 mL of a 0.2% solution of iron (III) chloride, and the absorbance at 390 nm was measured compared to the reference solution (2 mL of a 0.2% solution of FeCl3·6H2O). The reaction and measurements were conducted at a temperature of 25 °C ± 5 °C. Finally, the lactic acid concentration was calculated using a calibration curve that accounted for the 20-fold dilution of the Tej sample.
Volatile aromatic compound profiling
Volatile aromatic compounds were profiled using a headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS–SPME–GC–MS). Tej samples produced using various inoculum combinations were examined for volatile aromatic compounds by adapting the methods used by Ravasio et al.66. Briefly, 2.5 mL of the Tej sample and internal standard of 2-octanol (10 µL) was placed in a 20 mL head space extraction glass vials. Samples were then equilibrated for 10 min at 40 °C and 250 rpm. The extract was then adsorbed for 30 min at 40 °C using 50 µm layer DVB/CAR/PDMS fiber. After extraction, the fibers were drawn into the needle and transferred to the injection of the GC–MS system for desorption at 250 °C for 5 min. Chromatographic separation was performed with a TRACE TR-5 GC column (30 m,0.25 mm × 0.25 μm), using helium as carrier gas at 1.0 mL/min. The oven temperature program was set as follows: 50 °C to 220 °C, at 2 °C/min, then raised to 240 °C, at 10 °C /min and held for 10 min. The total run time was 91 min. The mass spectrometer (quadrupole) operated in full scan mode, detecting fragments in a mass range of 35 to 500 m/z. The Internal standard was used to semi-quantify volatile aromatic compounds following the method used by Pino and Barzola-Miranda67. The final identification of aromatic compounds was conducted by comparing mass spectra with the NIST 14 library and chemical standards.
Sensory analysis
The acceptability of Tej samples fermented by direct inoculation of isolated strains was assessed using consumer-based sensory analysis. Twenty-four experienced consumers were chosen to evaluate the sensory attributes of the test samples. The evaluations were conducted in a sensory panel room at 25 °C. About 10 mL Tej samples were served to the panelist using transparent plastic cup. The samples were evaluated twice after being blind-coded with three-digit random numbers. The panelists were then asked to rate each sample, with a seven-point hedonistic scale, for the (a) color (b) turbidity (c) alcoholic aroma (d) honey like aroma (e) astergency and (f) overall acceptance.
Statistical analysis
Except for the volatile compound analysis, which was done in duplicate, all other experimental analyses were done in triplicate. The collected data were then checked for normality and homoscedasticity (Levene’s test)68. Subsequently, means of the samples data were compared by Duncan’s multiple comparison test. The statistical significance (P < 0.05) was later determined by a one-way analysis of variance (ANOVA). The data analysis, CCA and PCoA plots were performed using RStudio 4.0.3 software.
Data availability
The datasets generated and/or analyzed during the current study are available in the National Center for Biotechnology Information (NCBI) repository with Accession Number PRJNA858936, and PRJNA858953.
References
Steinkraus, K. H. Industrialization of Indigenous Fermented Foods (Marcel Dekker, New York, 2004).
Lemi, B. Microbiology of Ethiopian traditionally fermented beverages and condiments. Int. J. Microbiol. 2020, 1–8 (2020).
Fentie, E. G., Emire, S. A., Demsash, H. D., Dadi, D. W. & Shin, J.-H. Cereal- and fruit-based ethiopian traditional fermented alcoholic beverages. Foods 9, 1–16 (2020).
Bahiru, B., Mehari, T. & Ashenafi, M. Yeast and lactic acid flora of tej, an indigenous Ethiopian honey wine: Variations within and between production units. Food Microbiol. 23, 277–282 (2006).
Vogel & Gobezie. Indigenous fermented foods in which ethanol is a major product. In: Hand book of Indigenous Fermented Foods 365–373 (Marcel Dekker Inc, 1995).
Bahiru, B., Mehari, T. & Ashenafi, M. Chemical and nutritional properties of `tej’, an indigenous Ethiopian honey wine: Variations within and between production units. Afr. J. Food Sci. 6, 104–108 (2001).
Fentie, E. et al. Physicochemical properties, antioxidant activities and microbial communities of Ethiopian honey wine, Tej. Food Res. Int. 152, 110765 (2022).
Nemo, R. & Bacha, K. Microbial, physicochemical and proximate analysis of selected Ethiopian traditional fermented beverages. LWT 131, 109713 (2020).
Fentie, E. G. et al. Fermentation dynamics of spontaneously fermented Ethiopian honey wine, Tej. LWT Food Sci. Technol. 155, 112927 (2022).
Verni, M., Verardo, V. & Rizzello, C. How fermentation affects the antioxidant properties of cereals and legumes. Foods 8, 362 (2019).
Svensson, L., Sekwati-Monang, B., Lutz, D. L., Schieber, A. & Gänzle, M. G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 58, 9214–9220 (2010).
Navarrete-Bolaños, J. L. Improving traditional fermented beverages: How to evolve from spontaneous to directed fermentation: Guidelines to design efficient directed fermentations based on spontaneous fermentations. Eng. Life Sci. 12, 410–418 (2012).
Barrajón, N., Capece, A., Arévalo-Villena, M., Briones, A. & Romano, P. Co-inoculation of different Saccharomyces cerevisiae strains and influence on volatile composition of wines. Food Microbiol. 28, 1080–1086 (2011).
Ciani, M., Comitini, F., Mannazzu, I. & Domizio, P. Controlled mixed culture fermentation: a new perspective on the use of non- Saccharomyces yeasts in winemaking. FEMS Yeast Res. 10, 123–133 (2010).
Diaz, M. et al. Comparison of the microbial composition of African fermented foods using amplicon sequencing. Sci. Rep. 9, 13863 (2019).
Djeni, T. N. et al. Microbial diversity and metabolite profiles of palm wine produced from three different palm tree species in côte d’ivoire. Sci. Rep. 10(1), 1–12 (2020).
Teramoto, Y., Sato, R. & Ueda, S. Characteristics of fermentation yeast isolated from traditional Ethiopian honey wine, ogol. Afr. J. Biotechnol. 4, 160–163 (2005).
Padilla, B., Gil, J. & Manzanares, P. Challenges of the non-conventional yeast Wickerhamomyces anomalus in winemaking. Fermentation 4, 1–14 (2018).
Lee, S.-B. & Park, H.-D. Isolation and investigation of potential non-saccharomyces yeasts to improve the volatile terpene compounds in korean muscat bailey a wine. Microorganisms 8, 1552 (2020).
Clemente-Jimenez, J. M., Mingorance-Cazorla, L., Martínez-Rodríguez, S., Las Heras-Vázquez, F. J. & Rodríguez-Vico, F. Influence of sequential yeast mixtures on wine fermentation. Int. J. Food. Microbiol. 98, 301–308 (2005).
Bauer, R. & Dicks, L. M. Control of malolactic fermentation in wine. A review. S. Afr. J. Enol. Vitic. 25(2), 74–88 (2004).
Escalante, A. et al. Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 235, 273–279 (2004).
Ayivi, R. D. et al. Lactic acid bacteria: Food safety and human health applications. Dairy 1, 202–232 (2020).
Mishra, S., Nanda, S., Madaan, N. & Mudgal, V. Microbial population succession in alcoholic beverages produced from some tropical fruits. Open Nutraceuticals J. 4 (2010).
Saxena, S., Verma, J. & Modi, D. R. RAPD-PCR and 16S rDNA phylogenetic analysis of alkaline protease producing bacteria isolated from soil of India: Identification and detection of genetic variability. J. Genet. Eng. Biotechnol. 12(1), 27–35 (2014).
Seseña, S., Sánchez, I. & Palop, L. Genetic diversity (RAPD-PCR) of lactobacilli isolated from “Almagro” eggplant fermentations from two seasons. FEMS Microbiol. Lett. 238(1), 159–165 (2004).
Legras, J.-L., Ruh, O., Merdinoglu, D. & Karst, F. Selection of hypervariable microsatellite loci for the characterization of Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 102, 73–83 (2005).
Saeed, A. F., Wang, R. & Wang, S. Microsatellites in pursuit of microbial genome evolution. Front. Microbiol. 6, 1462 (2016).
dos Santos Júnior, V. et al. Micronutrient requirements and effects on cellular growth of acetic acid bacteria involved in vinegar production. Food Sci. Technol. https://doi.org/10.1590/fst.05121 (2021).
Stubblefield, E. & Mueller, G. Effects of sodium chloride concentration on growth, bio chemical composition, and metabolism of hela cells. Am. J. Cancer Res. 20, 646–1655 (1960).
Lund, P. A. et al. Understanding how microorganisms respond to acid ph is central to their control and successful exploitation. Front. Microbiol. 11, 556140 (2020).
Sun, W. et al. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production. Metab. Eng. Commun. 10, e00124 (2020).
Peleg, M. & Corradini, M. G. Microbial growth curves: What the models tell us and what they cannot. Crit. Rev. Food Sci. Nutr. 51, 917–945 (2011).
Egli, T. Microbial growth and physiology: A call for better craftsmanship. Front. Microbiol. 6, 287 (2015).
Jin, X., Chen, W., Chen, H., Chen, W. & Zhong, Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as starter culture to produce mango Slurry: Microbiological, chemical parameters and antioxidant activity. Molecules 24, 4349 (2019).
Thomas, K. C., Hynes, S. H. & Ingledew, W. M. Effect of lactobacilli on yeast growth, viability and batch and semi-continuous alcoholic fermentation of corn mash. J. Appl. Microbiol. 90(5), 819–828 (2001).
Dysvik, A. et al. Co-fermentation involving saccharomyces cerevisiae and lactobacillus species tolerant to brewing-related stress factors for controlled and rapid production of sour Beer. Front. Microbiol. 11, 279 (2020).
Pereira, A. P., Mendes-Ferreira, A., Oliveira, J. M., Estevinho, L. M. & Mendes-Faia, A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 33, 114–123 (2013).
Basso, T. O. et al. Homo- and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Antonie Van Leeuwenhoek 105, 169–177 (2014).
Osburn, K. et al. Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol. 70, 76–84 (2018).
Peepall, C., Nickens, D. G., Vinciguerra, J. & Bochman, M. L. An organoleptic survey of meads made with lactic acid-producing yeasts. Food Microbiol. 82, 398–408 (2019).
Chitarrini, G. et al. Volatile profile of mead fermenting blossom honey and honeydew honey with or without ribes nigrum. Molecules 25, 1818 (2020).
Pereira, A. P. et al. Volatile composition and sensory properties of mead. Microorganisms 7, 1–16 (2019).
Erten, H., Tanguler, H. & Cakiroz, H. The effect of pitching rate on fermentation and flavour compounds in high gravity brewing. J. Inst. Brew. 113, 75–79 (2007).
Liu, S., Laaksonen, O. & Yang, B. Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol. 80, 25–39 (2019).
Panighel, A. & Flamini, R. Applications of solid-phase microextraction and gas chromatography/mass spectrometry (spme-gc/ms) in the study of grape and wine volatile compounds. Molecules 19, 21291–21309 (2014).
Begala, M., Corda, L., Podda, G., Fedrigo, M. A. & Traldi, P. Headspace solid-phase microextraction gas chromatography/mass spectrometry in the analysis of the aroma constituents of Cannonau of Jerzu wine. Rapid Commun. Mass Spectrom. 16, 1086–1091 (2002).
Ferreira, V., Lopez, R. & Cacho, J. F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 80, 1659–1667 (2000).
Pascoal, A., Anjos, O., Feás, X., Oliveira, J. M. & Estevinho, L. M. Impact of fining agents on the volatile composition of sparkling mead: Impact of fining agents on the volatile composition of sparkling mead. J. Inst. Brew. 125, 125–133 (2019).
Zhang, Y. et al. Aroma Characterization and Safety Assessment of a Beverage Fermented by Trametes versicolor. J. Agric. Food Chem. 63, 6915–6921 (2015).
Styger, G., Prior, B. & Bauer, F. F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 38, 1145–1159 (2011).
Nan, L. et al. Comparison of aroma compounds in cabernet sauvignon red wines from five growing regions in Xinjiang in China. J. Food Qual. 2021, 1–16 (2021).
Tao, Y. et al. Chemical composition and sensory profiles of mulberry wines as fermented with different Saccharomyces cerevisiae strains. Int. J. Food Prop. https://doi.org/10.1080/10942912.2017.1361970 (2017).
Englezos, V. et al. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 257, 350–360 (2018).
Wang, L. et al. Suppression of volatile production in tomato fruit exposed to chilling temperature and alleviation of chilling injury by a pre-chilling heat treatment. LWT Food Sci. Technol. 62, 115–121 (2015).
Verzera, A. et al. Volatile compound and sensory analysis for the characterization of an italian white wine from “inzolia” grapes. Food Anal. Methods 1, 144–151 (2008).
Qvirist, L. A. et al. Isolation, identification and characterization of yeasts from fermented goat milk of the yaghnob valley in tajikistan. Front. Microbiol. 7, 1690 (2016).
Saarisalo, E., Skyttä, E., Haikara, A., Jalava, T. & Jaakkola, S. Screening and selection of lactic acid bacteria strains suitable for ensiling grass. J. Appl. Microbiol. 102(2), 327–336 (2007).
Reiner, K. Carbohydrate fermentation protocol. American Scociety for Microbiology (2012).
Jermini, M., Geiges, O. & Schmidt-Lorenz, W. Detection, isolation and identification of osmotolerant yeast from high-sugar products. J. Food Prot. 50, 468–472 (1987).
Kostinek, M. et al. Diversity and technological properties of predominant lactic acid bacteria from fermented cassava used for the preparation of Gari, a traditional African food. Syst. Appl. Microbiol. 28, 527–540 (2005).
Vaudano, E. & Garcia-Moruno, E. Discrimination of Saccharomyces cerevisiae wine strains using microsatellite multiplex PCR and band pattern analysis. Food Microbiol. 25, 56–64 (2008).
Ma, C., Sun, Z., Chen, C., Zhang, L. & Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 145, 784–788 (2014).
OIV. The International Organisation of Vine and Wine. Compendium of international methods of wine and must analysis. vol. 1 (2020).
Borshchevskaya, L. N., Gordeeva, T. L., Kalinina, A. N. & Sineokii, S. P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 71, 755–758 (2016).
Ravasio, D. et al. Adding flavor to beverages with non-conventional yeasts. Fermentation 4, 15 (2018).
Pino, J. A. & Barzola-Miranda, S. E. Characterization of odor-active compounds in pechiche (Vitex cymosa Berteo ex Speng) fruit. J. Raw Mater. Process. Foods 1, 7 (2020).
Granato, D., de Araújo Calado, V. M. & Jarvis, B. Observations on the use of statistical methods in food science and technology. Food Res. Int. 55, 137–149 (2014).
Acknowledgements
One of the authors, Eskindir Getachew Fentie, would like to acknowledge Addis Ababa Science and Technology University, Addis Ababa University, and Kyungpook National University. Besides, we would like to acknowledge all of the panelists who participated in the sensory analysis. Furthermore, National Research Foundation of Korea (Grant No. 2020R1I1A307452212), and Korea Basic Science Institute (Grant No. 2021R1A6C101A416) are also duly acknowledged.
Author information
Authors and Affiliations
Contributions
E.G.F: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Visualization, Writing–original draft. M-S.J: Investigation, Software, Visualization. S.A.E: Conceptualization, Writing—review & editing, Supervision. H.D.D: Conceptualization, Writing—review & editing, Supervision. M-C.K:, Investigation K.L: Investigation. J-H. S: Conceptualization, Funding acquisition, Writing—review & editing, Resources, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fentie, E.G., Jeong, M., Emire, S.A. et al. Development of mixed starter culture for the fermentation of Ethiopian honey wine, Tej. Sci Rep 12, 13431 (2022). https://doi.org/10.1038/s41598-022-17594-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17594-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.