Abstract
The Opuntia ficus-indica (L.) cactus, a crucial crop in Morocco, is threatened by the wild cochineal, Dactylopius opuntiae (Cockerell). The aim of this research was to investigate the efficacy of nine bacterial strains against both D. opuntiae nymphs and adults females applied individually or after black soap in the laboratory, greenhouse, and field conditions. Using the partial 16S ribosomal DNA, the bacterial isolates were identified as Pseudomonas koreensis, Pseudomonas sp., Burkholderia sp. and Bacillus sp. Under laboratory conditions, the insecticidal activity of P. koreensis strain 66Ms.04 showed the level mortality (88%) of adult females’ at 108 CFU/mL, 7 days after application. At a concentration of 108 CFU/mL, P. koreensis strain 66Ms.04 and Pseudomonas sp. (strains 37 and 5) caused 100% nymphs mortality rate three days after application. Under greenhouse conditions, the use of P. koreensis strain 66Ms.04 at 108 CFU/mL following the application of black soap (60 g/L) demonstrated the maximum levels of females and nymphs’ mortalities with 80 and 91.25%, respectively, after 8 days of treatment. In field conditions, the combined application of the P. koreensis strain 66Ms.04 at 108 CFU/mL with black soap at 60 g/L, for an interval of 7 days, significantly increased the mortality of adult females to 93.33% at 7 days after the second application. These findings showed that the combined treatment of P. koreensis strain 66Ms.04 with black soap can be a potent and eco-friendly pesticide against D. opuntiae.
Similar content being viewed by others
Introduction
Opuntia ficus-indica (L.) Mill. (Caryophyllales: Cactaceae) commonly called prickly pear or nopal cactus, belongs to the dicotyledonous angiosperm family Cactaceae and originates from Mexico. This species has the ability to thrive in arid and semi-arid environments and geographically distributed in South Africa, Latin America, and the Mediterranean countries1. It has special adaptive mechanisms and a high biomass production capacity, which allows it to grow in adverse conditions, such as high temperatures and nutritionally poor soils subject to erosion2. The cactus has been present in Morocco since 1770 and it’s currently widely distributed in the national landscape. As a result of drought, the cactus area has expanded significantly over the past twenty years, it has increased from about 50,000 ha in 1998 to more than 150,000 ha in 20173. Cactus pear is considered as great source of food and feed. It has been traditionally recognized as a nutrient that can provide valuable health benefits, in addition to its wide range of uses in the pharmaceutical industry4. The modernization of Moroccan agriculture (Green Generation Plan) encourages cactus plantation as an alternative crop in less favorable regions3. However, the crop has been suffering from the attack of a sap-sucking insect pest, the wild cochineal Dactylopius opuntiae (Hemiptera: Dactylopiidae) since 2014. The rapid and aggressive spread of the cochineal pest in various regions of Morocco has led to significant socio-economic consequences5. This pest is widely distributed throughout the Mediterranean basin and has become a serious threat to the prickly-pear crop6. According to Ochoa et al.7, D. opuntiae is a pest that is present in more than 30 countries where cactus is cultivated. Both nymphs and adult females of D. opuntiae suck sap from the cladodes of the plants, leading to their desiccation, weakening, and death8. The females of the wild cochineal have a white waxy coating that provides a physical barrier against predators and helps them to maintain an ideal moisture level.
Significant progress has been achieved in applying of the integrated pest management approach using a combination of techniques such as planting cochineal-resistant host plants, biological control and the use of biopesticides derived from natural sources to effectively manage the wild cochineal population in Morocco5, 9,10,11. Over the last two decades, the use of synthetic chemicals has led to several environmental problems and health risks12. Some chemical pesticides have already been banned by the EU and the US due to environmental and human health problems13.
Chemical insecticides have played a major role in the control of insect pests. However, the growing demand to reduce synthetic chemicals use due to environmental and human health concerns, in addition to pesticide resistance issues, is fuelling interest in innovative and sustainable approaches to manage this new invasive cochineal D. opuntiae.
Biological control with entomopathogenic fungi14 and entomopathogenic bacteria15 offers a better alternative to synthetic chemical pesticides, because of the high specificity of the biopesticides, their easy biodegradability, their short shelf-life and environmental friendly for sustainable agriculture16, 17. Many microbial pathogens of insects are intensively investigated to develop environmentally friendly pest management strategies in agriculture18. Over 100 bacterial species with entomopathogenic activity have been identified as both exo- and endo-pathogens of arthropods18, 19. But only some of these bacterial entomopathogens are commercially available for agricultural uses. Some of the bacteria used commercially are: Bacillus thuringiensis, Bacillus Lysinibacillus, Bacillus popilliae, Pseudomonas alcaligenes, Clostridium bifermentans, Saccharopolyspora spinosa, Pseudomonas aureofaciens, Streptomyces avermitilis and Serratia entomophila were the most studied20,21,22. In a previous research, Idris et al.15 showed the potential of crude enzymes produced by Bacillus subtilis, the local strain SY134D, to control D. opuntiae insects under laboratory conditions.
The aim of the present investigation is to study the insecticidal effect of different bacterial strains isolated from Moroccan soils for the management of nymphs and females of D. opuntiae in the laboratory, greenhouse, and field conditions. The findings of this study will be exploited in the development of microbial insecticide formulation, which can effectively protect the prickly pear from the scale insect D. opuntiae as an eco-friendly, target‐specific, easily biodegradable, and safer alternative agricultural product.
Materials and methods
Isolation of the bacterial strains
The bacterial strains used in this work were isolated from a set of soils belonging to different regions of Morocco. Table 1 shows the geographical locations and site details of sampled soils. The soil samples were collected at a depth of 30 cm close to the roots. The soils were placed in sterile polypropylene bags and immediately transported to the laboratory. The soil samples were stored at 4°C and processed within 48 h by examining 0.1 g of a subsample from each sample. The subsamples were transferred and homogenized in 1 mL of sterile physiological water. Soil suspensions were serially diluted (from 10–2 to10–9) and aliquots were placed on Burk's agar plates using a 100 µL spreader23, then incubated for 4–5 days at 30 °C. After that, single colonies were preserved in Burk's agar medium for additional purification. For long-term storage, each isolate was kept at -80°C in liquid Burk's medium, which contains 30% (v/v) glycerol.
Identification of the bacterial strains
The isolated bacterial strains were identified based on partial 16S ribosomal DNA (16S rDNA). The genomic DNA of bacterial strains was extracted by Pure- Link™ Genomic DNA Mini Kit (Invirogen, K182001). PCR reactions were performed using DreamTaq DNA Polymerase PCR Master Mix comprising 1 μg DNA, 0.4 mM dNTPs, 4 mM MgCl2 (Invirogen, K1071), and 1 μM of each of the following primers 27F 5′-AGA GTT TGA TCC TGG CTC AG-3′/1492R 5′- ACG GTT ACC TTG TTA CGA CTT-3′24were used to amplify the 16S rDNA, in a final reaction volume of 25 μL. The process of thermocycling involved subjecting the sample to different temperatures in a PCR machine. The first step was DNA denaturation at 95 °C for 1 min, followed by the annealing with 35 cycles of 95 °C for 30 s, 53 °C for 30 s, and 72 °C for 1 min. Finally, the sample was subjected to a terminal extension at 72 °C for 15 min. The resulting PCR products were verified using a 1% agarose gel and purified using the PureLink Quick Gel Extraction Kit from Invitrogen (K220001). The PCR products were then transferred to Secugen S.L. (https://www.secugen.es) for sequencing, and the obtained sequences were compared with those available in the NCBI server (https://blast.ncbi.nlm.nih.gov/Blast.cgi)25 and submitted in GenBank (Table 2). To determine the evolutionary relationships between the sequences, a phylogenetic tree was constructed using the neighbor-joining method in the MEGA 7.0 software.
Preparation of bacterial suspensions
The bacterial stock was initiated from a single colony of each of the eight bacteria, inoculated in liquid media Luria–Bertani Broth (LB), and grown for 48 h at 25 ± 2 °C in darkness under agitation at 150 rpm. The optical densities of bacterial suspensions were determined using UV/VIS spectrophotometer (T80, PG-Instruments) and the populations of cells at the various optical densities were determined by dilution plating.
Laboratory bioassays
Insect rearing
One-year-old healthy young cladodes of O. ficus-indica, were planted in plastic pots measuring 27 cm in diameter and 24 cm in height. The pots were filled with a soil mixture consisting of equal parts of sand, peat, and soil, with a volume ratio of 1:1:1. Healthy young cladodes were first placed in a glasshouse at 30 ± 5°C where they were subjected to a heavy infestation of cladodes collected from the Rabat region of Morocco (33°59′57″ N 6°23′27″ W). All cactus cladodes used in the trials, were collected in conformity with Moroccan Agriculture Ministry guidelines and regulations. Each cladode previously infested with the D. opuniae was placed in two pots to infest the healthy cladodes. After exposing the cladodes to the infested colonies for a month, the colonies with adult females were selected to be used in different experiments.
Contact toxicity
The immersion application method was used to assess the contact toxicity of eight bacterial strains. The study was carried out in the laboratory under controlled conditions of 24 ± 2 °C temperature, 75% humidity, and a 14:10 (light:dark) photoperiod. Three concentrations (106, 107 and 108 CFU/mL) were specifically selected on the basis of preliminary tests, and were used mixing the bacterial strains with water.
Adult females of D. opuntiae
Ten first instar–mature females D. opuntiae of the same age were immersed in different bacterial strains at different concentrations for five seconds and deposited separately, using an entomological brush, on cladodes of the same size placed in Petri dishes (9 cm in diameter). The control adult females were immersed in water. The experiments were performed using a completely randomized design (CRD) with five replicates. The number of dead adult females was recorded every 24 h for a period of 8 days after the use of various treatments, using a binocular microscope (MoticDM-143). The dead females showed a dark brown color, and their bodies were desiccated.
Nymphs of D. opuntiae
Ten first instar nymphs of D. opuntiae of the same age (21 h) were deposited on cladodes of the same size placed in Petri dishes and were directly sprayed with different bacterial strains of different concentrations. The control nymphs were sprayed with distilled water. The bioassays were done using a completely randomized design (CRD) with five replicates. Mortality of nymphs was recorded every 24 h for a period of 8 days. The dead nymphs showed no movement and had dye modifications.
Toxicity of different bacterial strains alone or in combination with Black Soap under greenhouse conditions
The insecticidal activity of four bacterial strains (the most effective bacterial strains selected from laboratory tests) was tested alone or in combination with the black soap by contact application. The bacterial strains were used at a concentration of 108 CFU/Ml, while black soap with a concentration of 60 g/L was applied to facilitate the degradation of cuticular wax5 and then exposed the females to different bacterial strains. The bioassays were conducted in a completely randomized design (CRD) with four repetitions. The experimental procedure involved the application of the soap solution to cladodes first, followed by the application of bacterial strains using a 1L hand sprayer. Mortality rates of nymphs and females were recorded every 24 h for a period of 8 days after treatment. The application was decided at the medium level of infestation (26–50%) using Silva`s modified rating scale26 to determine the severity of wild scale infestation in cactus pear plants as follows: 0—not infested 0%; 1—low infestation 1–25%; 2—medium infestation 26–50%; 3—high infestation of 51–75%; 4—extensive infestation of 76–100%.
Field bioassay
The bacterial strains that exhibited considerable toxicity against nymphs and females of D. opuntiae in the laboratory and greenhouse conditions were chosen to evaluate their effectiveness in the field conditions from October to November 2021.
The field experiment was carried out near Rabat region, Morocco (33°59′57″ N 6°23′27″ W). The experimental design adopted a randomized complete block with each treatment repeated three times. In each plot, three cladodes were selected for treatment. The treatments consisted of applying the P. koreensis strain 66Ms.04 at a concentration of 108 CFU/mL either alone on the cladodes, or on cladodes that had been previously sprayed with black soap at a concentration of 60 g/L which served to remove the cuticular wax and exposed the females and nymphs to the used bacterial strain. Two different controls were used, the first control is cladodes treated with water only, and the second control is cladodes treated with black soap at 60g/L. The tested bacterial strain solutions were combined with the 0.01% of Triton X-100-stabilized emulsion used to improve the solubility and dispersion of the bacteria with water before application using a 2L hand sprayer, with a 250 l/ha rate and 8 ml min−1 frequency. The second spray was done seven days after the first one. Mortality of nymphs and adult females was recorded 3, 5 and 7 days of the first and second sprays. The application was decided at the low to medium levels of (26–50%) infestation using Silva`s modified rating scale (1991).
Statistical analysis
Before performing statistical analysis, mortality percentages were transformed into angular values (arcsine √P). In the laboratory, the transformed percentages were analysed using a two-way analysis of variance (ANOVA) to investigate the effects of bacterial strain concentrations and bacterial strain source. In order to estimate the lethal concentration for 50 and 90% mortality (LC50 and LC90 respectively), intercept, slope of the regression line, and fiducial limits, concentration-mortality, data was subjected to probit analysis27 using IBM SPSS Statistics 27.0. In the greenhouse and field experiments, the transformed percentages were subjected to a one-way ANOVA. To compare means, Tukey’s test was employed at a significance level of P < 0.05. All statistical analyses were conducted using Genstat (21st Edition, VSN International, Hemel Hempstead, UK).
Results
Identification of the bacterial strains
The isolates were determined via analysis of PCR-amplified 16S rDNA sequences as shown in Table 2. Using the NCBI database, the acquired sequences deposited in Genbank and were compared to the sequences already present in the databases. Most of the obtained strains were affiliated to the Pseudomonas sp. Only two strains named 41Ms and 18Ms were affiliated to Bacillus sp. and Burkholderia sp., respectively (Fig. 1).
Laboratory bioassays
The mortality of nymphs and adult females of D. opuntiae after exposure to different bacterial strains is presented in Tables 2 and 3. Data analysis showed a significant difference (p < 0.001) in mortality of D. opuntiae nymphs and adult females, caused by the nine bacteria strains at different tested concentrations for various exposure times. Two days after application, 100% mortality of nymphs was recorded for P. koreensis strain 66Ms.04, P. koreensis strain 37Ms and strain 5Ms at 108 CFU/mL. In 3 days, post-application, P. koreensis strain 37Ms and Pseudomonas sp. strain 5Ms at 108 (CFU/mL) reached 100% mortality of nymphs, followed by Pseudomonas sp. 27Ms (98%) at 108 (CFU/mL). While the lowest percentage mortality (14%) of nymphs was recorded for Bacillus sp. 41Ms at 106 (CFU/mL) (Table 3). The mortality of nymphs exposed to various exposure times increased noticeably as the tested bacteria’s concentrations increased.
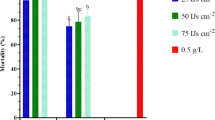
The results of the statistical data revealed that all the tested bacteria had significantly different mortality rates for adult females at various exposure times (p < 0.001; Table 4). The maximum rate of females mortality was recorded for P. koreensis strain 66Ms.04 and Pseudomonas sp. strain 37Ms at 108 (CFU/mL) with 50 and 46%, respectively four days after treatment. Seven days after application, P. koreensis strain 66Ms.04 at 108 CFU/mL showed the highest levels of adult females’ mortality (88%) (Fig. 2), followed by both Pseudomonas sp. strain 37Ms and Pseudomonas sp. strain 5Ms with 72% of adult mortality. However, the lowest mortality was recorded by Bacillus sp. 41Ms at 106 (CFU/mL) with 6%, 8 days after application (Table 4).
Greenhouse bioassay
The effects of four bacterial strains on D. opuntiae nymphs and adult females' mortality are presented in Tables 4 and 5. The ANOVA showed significant differences in nymphs mortality induced by various treatments and the checks at different exposure times (Fig. 3). Three days after treatments, P. koreensis strain 66Ms-04 and Pseudomonas sp. 27Ms and P. koreensis strain 37Ms and 5Ms strains applied at 108 CFU/mL before application of the black soap (60g/L) produced the maximum mortality of nymphs among all tested bacteria, with 80.0% mortality, respectively. For various bacterial strains, nymphs mortality increased with increasing concentrations for various exposure durations. The nymph’s mortalities increased significantly at the 8th day after application to reach its maximum for P. koreensis strain 66Ms-04 in combination with black soap (91.25%), followed by P. koreensis strain 66Ms-04 applied alone with 85% (Fig. 3).
Mean percentage ± SE of Dactylopius opuntiae nymphs’ mortality after treatment with various bacterial strains under greenhouse condition. PK66 + Bs: Pseudomonas koreensis strain 66Ms-04 + Black soap; PK66: Pseudomonas koreensis strain 66Ms-04; PS27 + Bs: Pseudomonas sp. 27Ms + Black soap; PS: Pseudomonas sp. 27Ms; PK37 + Bs: Pseudomonas koreensis strain 37Ms + Black soap; PK37: Pseudomonas koreensis strain 37Ms; PS5 + Bs: Pseudomonas sp. 5Ms + Black soap; PS5: Pseudomonas sp. 5Ms; Bs: Black soap. The different letters indicate significant differences between groups based on Tukey test (p < 0.05).
The ANOVA revealed a significant difference in the mortality of adult females of D. opuntiae induced by different bacterial strains and their application with black soap for different exposure periods (p < 0.001; Fig. 4). The mortality of adult females for different bacterial isolates increased with increasing concentrations for different exposure times. In 6 days after application, P. koreensis strain 66Ms-04 at (108 CFU/mL) combined with black soap (60 g/L) demonstrated the high level of mortality of adult females’ with 75.00%. The highest levels of D. opuntiae adult females’ mortality (80%) was recorded by application of P. koreensis strain 66Ms-04 (108 CFU/mL) in combination with black soap, 8 days after application, followed by P. koreensis strain 66Ms-04 applied alone (66.75%) (Fig. 4).
Mean percentage ± SE of Dactylopius opuntiae adult females’ mortality after treatment with various bacterial strains under greenhouse condition. PK66 + Bs: Pseudomonas koreensis strain 66Ms-04 + Black soap; PK66: Pseudomonas. koreensis strain 66Ms-04; PS27 + Bs: Pseudomonas sp. 27Ms + Black soap; PS: Pseudomonas sp. 27Ms;PK37 + Bs: P. koreensis strain 37Ms + Black soap;PK37: Pseudomonas koreensis strain 37Ms; PS5 + Bs: Pseudomonas sp. 5Ms + Black soap; PS5: Pseudomonas sp. 5Ms; Bs: Black soap. The different letters indicate significant differences between groups based on Tukey test (p < 0.05).
Probit analysis of the entomopathogenic bacteria effect shows that lethal concentrations (LC) varied between the tested bacterial strains. The estimated LC values of strains against D. opuntiae nymphs showed that three days after treatment, P. koreensis strain 66Ms-04, recorded LC50 = 1.23 × 106 and LC90 = 2.15 × 107, followed by Pseudomonas sp. 5Ms (LC50 = 1.37 × 106 and LC90 = 2.97 × 107) (Table 5). While the LC values of strains against D. opuntiae females indicated that P. koreensis strain 66Ms-04, (LC50 = 8.84 × 106 and LC90 = 1.70 × 108) were more effective than Pseudomonas sp. 5Ms (LC50 = 1.23 × 107 and LC90 = 3.53 × 108) eight days after treatment (Table 6). On the other hand, when the mortality rates of females were recorded for all four bacterial strains after each exposure time, the LC50 values could not be calculated for any of these strains, as none of the concentrations tested resulted in mortality rates higher than 50%.
Field bioassays
The mortality of D. opuntiae nymphs and adult females after exposure to the most effective bacterial strain is presented in Table 7. The statistical analysis using ANOVA revealed a significant difference in mortality rates of nymphs and females of D. opuntiae when exposed to P. koreensis strain 66Ms-04 and when used before the application of black soap for varying durations in the first and second treatment under field conditions (p < 0.01; Table 7). On the 3rd days after the first treatments, P. koreensis strain 66Ms-04 at 1 × 108 CFU/mL in combination black soap (30 g/L) and black soap applied alone showed the highest mortality rates of nymphs with 80 and 76.66%, respectively. Seven days after the first treatments, the mortality rates did not change significantly for both treatments, except for P. koreensis strain 66Ms-04 applied alone that reached 20% mortality. Five days after the second application, the nymph mortality increased to 93.33% for P. koreensis strain 66Ms-04 before treatment with black soap (60 g/L). However, the application of this bacterial strain alone reached only 50% mortality.
The ANOVA analysis indicated a statistically significant difference in the mortality of females of D. opuntiae produced by different treatments, performed for varying exposure times during the first and second treatment (p < 0.01; Table 7). The mortality rate of female adults increased to 60%, 7 days after the first treatment by P. koreensis strain 66Ms-04 (108 CFU/mL) applied with black soap. Seven days after the second application of P. koreensis strain 66Ms-04 applied with black soap, the mortality rate of adult females considerably increased to reach 78%.
Discussion
Plant growth promoting (PGP) bacteria are well known for their usefulness in crop production and protection and in maintaining soil quality. In the local context of Morocco, the use of local antagonistic PGP bacteria including Bacillus spp., Pseudomonas spp., have been cited not only to improve plant growth, but also as a possible eco-friendly alternative to control insect pests and plant pathogens28, 29. In the present study, we evaluated the insecticidal potential of various bacterial strains applied alone and combined with a detergent for the control of D. opuntiae nymphs and adult females. Among the different microbial strains tested to control D. opuntiae at various stages, the best results were achieved with a double application of P. koreensis strain 66Ms-04 at a concentration of 108 CFU/mL, in combination with black soap at a concentration of 60 g/L. The results showed that the insecticidal activity of different bacterial strains was found to increase with increasing concentrations and exposure times under laboratory conditions. However, the insecticidal effect of the P. koreensis strains exhibited greater efficacy when used in combination with black soap, without causing any noticeable harm to the treated plants. Many authors have identified more than 100 bacterial species with entomopathogenic activity18, 30,31,32.
Among the genera of entomopathogenic bacteria most used in the management of various insect pests, we found Bacillus, Pseudomonas, Lysinibacillus, Serratia and Chromobacterium, Xenorhabdus, Photorhabdus33,34,35. Among the bacteria used for commercial purposes, L. sphaericus, B. popilliae, C. bifermentans, B. thuringiensis, P. alcaligenes, S. spinosa, Pseudomonas aureofaciens, S. avermitilis and S. entomophila were considered the most used and appreciated microbial pest control agents.
The genus Pseudomonas belongs to the Gammaproteobacteria, a class of bacteria that emerged from the Hydrobacteria 1.75 billion years ago36, and belonging to the family of Pseudomonadaceae which has been studied extensively and has over 200 described species37. Pseudomonas is one of the most ubiquitous genera in the world; they are founded in environmental habitats such as the soil38, the surface of plants39, 40and the guts of insects41. These bacteria are very adaptive and capable to use a large range of compounds as an energy source. There are many species that occur in association with plants and animals, mainly as saprophytes, but some are also pathogenic to them42.
Pseudomonas bacteria have beneficial applications in biotechnology, in the promotion, of plant growth, in bioremediation and in biological control43. Species of pseudomonads that are pathogenic to insects include, Pseudomonas aeruginosa, Pseudomonas protegens, Pseudomonas chlororaphis, Pseudomonas fuorescens, Pseudomonas putida, Pseudomonas entomophila, Pseudomonas taiwanensis, Pseudomonas mosselli, Pseudomonas syringae, and several more strains of Pseudomonas spp.44,45,46,47,48,49,50,51,52,53.
The insecticidal properties of P. koreensis have been documented in various studies, indicating its ability to effectively control a broad spectrum of insect pests. P. koreensis is a gram-negative bacteria, first described as a new species by Kwon et al.54. The species was obtained from a Korean agricultural soil with low pH and can to grow at 4 °C.
Hultberg et al.55 showed that P. koreensis 2.74 (CBS 125413) produces the Cyclic lipopeptides (CLP) lokisin and a crude extract of this CLP with a protective effect against tomato disease Pythium ultimum. The same search also revealed that P. koreensis 2.74 and the CLP significantly reduce potato late blight disease induced by Phytophthora infestans in a detached-leaf test56.
Ruffner et al.48 demonstrated that Fit toxin producing Pseudomonas exhibit potent oral activity against larvae of Spodoptera littoralis (Lepidoptera: Noctuidae), Chloridea virescens (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). Spraying plant leaves with suspensions containing only 1000 Pseudomonas cells per ml was sufficient to kill 70–80% of Spodoptera and Helicoverpa larvae.
In addition, the study of Rangel et al.57 showed that the three strains within the P. chlororaphis subgroup exhibited both oral and injectable toxicity to the tobacco hornworm Manduca sexta (Lepidoptera: Sphingidae). The three strains possess the gene cluster encoding for the insect toxin FitD. The same authors reported that P. protegens Pf-5 exhibited substantial levels of oral toxicity against the dipteran Drosophila melanogaster (Diptera: Drosophilae)57. A number of P. fluorescens strains and P. protegens Pf-5 have been shown to kill or to cause morphologic defects in D. melanogaster adult flies that emerged from the infected larvae57, 58.
In the present study, the black soap detergent at 60 g/L was employed to remove the thicker wax, making female and nymph D. opuntiae more susceptible to the strong contact toxicity of the bacterial strain tested. Black soap is a natural product produced from fatty acids obtained from olive oil. Secondary metabolite production in Pseudomonas has been reviewed extensively. It has become evident that only a limited number of bioactive compounds play a clear role in biocontrol of plant diseases such as hydrogen cyanide (HCN), 2,4-diacetylphloroglucinol (DAPG); phenazines, pyrrolnitrin, pyoluteorin, 2-hexyl-5-propyl-alkylresorcinol, siderophores; and (cyclic) lipopeptides42. In addition, Lin et al.59 reported that P. koreensis CRS05-R5 exhibited a biocontrol effect against Sitophilus oryzae (Coleoptera: Curculionidae) and Acidovorax avenae subsp. avenae. The study of Ichikawa et al.60 showed that P. koreensis CRS05-R5 genome had more than 800 genes predicted to be involved in secondary metabolism. The present study showed that both Bacillus species (Bacillus sp. 41Ms and B. thuringiensis subsp. kurstaki ABTS-351) did not show a good efficacy to control the mature females. However, the Bacillus sp. 41Ms species resulted in moderate mortality against nymphs 3 days after application. In contrast, Idris et al.15 reported a significant insecticidal effect against both nymphs and females of D. opuntiae using the crude enzyme solution produced by Bacillus subtilis SY134D strain at concentration 100%. This strain of B. subtilis SY134D used reported as a good producer of chitinase and lipase and other six hydrolytic enzymes. The author suggests that the death of nymphs and adults mature could be attributed to cochineal wax hemolysis by the lipase and then chitin degradation by chitinase.
Conclusions
The results of this study suggest that the combination of double applications of the bacterial strain P. koreensis 66Ms-04 at 108 CFU/mL with black soap at 60 g/L could be used as a component of integrated pest management (IPM) for controlling D. opuntiae. This approach provides an effective and environmentally friendly alternative to chemical insecticides. However, further research is necessary to understand the mechanisms and identify the causes of toxicity against different stages of D. opuntiae, by identifying the responsible bacterial metabolites, enzymes, their combinations. Through this process, researchers may be able to identify the most efficient and targeted way to use these bacteria to manage the cochineal insect.
The interaction of these pseudomonads with other biocontrol agents, which could have a synergistic effect on wild cochineal control, can also be studied. These findings showed that entomopathogenic bacteria are promising for developing a biopesticide formulation for the control of D. opuntiae as an effective and safe alternative to pesticides.
Patents
One patent resulting from the work reported in this manuscript. The patent titled: Bacterial strain of Pseudomonas koreensis and an insecticide composition for the control of the wild cochineal Dactylopius opuntiae. Patent registered in the Moroccan office of industrial and commercial property (OMPIC) under number 5794.
Data availability
The data is available on request from the corresponding author (KEL).
References
Butera, D. et al. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica ) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 50, 6895–6901. https://doi.org/10.1021/jf025696p (2002).
Jaiswal, A. Nutritional Composition and Antioxidant Properties of Fruits and Vegetables, 1st edn. https://www.elsevier.com/books/nutritional-composition-and-antioxidant-properties-of-fruits-and-vegetables/jaiswal/978-0-12-812780-3 (2020).
MAPMDREF. http://www.agriculture.gov.ma/pages/actualites/cochenille-du-cactus-lancement-d%E2%80%99une-assistance-technique-de-la-fao-pour-l%E2%80%99eradicat (2017).
Shetty, A. A., Rana, M. K. & Preetham, S. P. Cactus: A medicinal food. J. Food Sci. Technol. 49, 530–536. https://doi.org/10.1007/s13197-011-0462-5 (2012).
Ramdani, C. et al. Chemical composition and insecticidal potential of six essential oils from morocco against Dactylopius opuntiae (Cockerell) under field and laboratory conditions. Insects 2075–4450(12), 1007–1007. https://doi.org/10.3390/insects12111007 (2021).
Mazzeo, G., Nucifora, S., Russo, A. & Suma, P. Dactylopius opuntiae, a new prickly pear cactus pest in the Mediterranean: An overview. Entomol. Exp. Appl. 167, 59–72. https://doi.org/10.1111/eea.12756 (2019).
Ochoa, M. J. & Barbera, G. History, economic and agro-ecological importance. In Crop Ecology Cultivation and Uses of Cactus Pear (eds Inglese, P., Mondragon, C., Nefzaoui, A. & Sáenz, C.) (Food and Agriculture Organization of the United Nations and the International Center for Agricultural Research in the Dry Areas, Rome, 2017).
Vanegas-Rico, J. M. et al. Enemigos naturales de Dactylopius opuntiae (Cockerell) en Opuntia ficus-indica (L.) Miller en el centro de Mexico. Acta Zool. Mex. 26, 415–434. https://doi.org/10.21829/azm.2010.262718 (2010).
Bouharroud, R., Sbaghi, M., Boujghagh, M. & El Bouhssini, M. Biological control of the prickly pear cochineal Dactylopius opuntiae Cockerell (Hemiptera: Dactylopiidae). EPPO Bull. 48, 300–306. https://doi.org/10.1111/epp.12471 (2018).
Sbaghi, M., Bouharroud, R., Boujghagh, M. & Bouhssini, M. E. Sources de résistance d’Opuntia spp. contre la cochenille à carmin Dactylopius opuntiae, au Maroc. EPPO Bull. 49, 585–592. https://doi.org/10.1111/epp.12606 (2019).
El Aalaoui, M. et al. Comparative toxicity of different chemical and biological insecticides against the scale insect Dactylopius opuntiae and their side effects on the predator Cryptolaemus montrouzieri. Arch. Phytopathol. Plant Prot. 52, 155–169. https://doi.org/10.1080/03235408.2019.1589909 (2019).
Aktar, W., Sengupta, D. & Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2, 1–12. https://doi.org/10.2478/v10102-009-0001-7 (2009).
House, M. & Green, P. Assessment of the impact on crop protection in the UK of the ‘cut-off criteria’ and substitution provisions in the proposed Regulation of the European Parliament and of the Council concerning the placing of plant protection products in the market. Pesticides Safety Directorate, UK (2008).
Ramdani, C. et al. Entomopathogenic fungi as biological control agents of Dactylopius opuntiae (Hemiptera: Dactylopiidae) under laboratory and greenhouse conditions. Front. Sustain. Food Syst. 6, 997254. https://doi.org/10.3389/fsufs.2022.997254 (2022).
Idris, I., Elkhouri, S. & Bakri, Y. Evaluation of crude enzyme produced by Bacillus subtilis SY134D culture as a biocontrol agent against Dactylopius opuntiae (dactylopiidae: hemiptera) on cactus pear. J. Innov. 8, 289–300 (2019).
Sayyed, R. Z. & Patel, P. R. Biocontrol potential of siderophore producing heavy metal resistant Alcaligenes sp. and Pseudomonas aeruginosa RZS3 vis-à-vis organophosphorus fungicide. Indian J. Microbiol. 51, 266–272. https://doi.org/10.1007/s12088-011-0170-x (2011).
Kumar, S. & Singh, A. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 6, 1–2. https://doi.org/10.4172/2471-2728.1000e129 (2015).
Chattopadhyay, P., Banerjee, G. & Mukherjee, S. Recent trends of modern bacterial insecticides for pest control practice in integrated crop management system. 3 Biotech 7, 60. https://doi.org/10.1007/s13205-017-0717-6 (2017).
Kalha, C. S. et al. Entomopathogenic viruses and bacteria for insect-pest control. In Integrated Pest Management: Current Concepts and Ecological Perspective 225–244 (Academic Press, 2014).
Johnson, V. W., Pearson, J. & Jackson, T. A. Formulation of Serratia entomophila for biological control of grass grub. N. Z. Plant Prot. 54, 125–127. https://doi.org/10.30843/nzpp.2001.54.3752 (2001).
Roh, J.-Y., Choi, J.-Y., Li, M.-S., Jin, B.-R. & Je, Y.-H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 17, 547–559 (2007).
Jeong, H. U. et al. Evaluation of insecticidal activity of a bacterial strain, Serratia sp. EML-SE1 against diamondback moth. J. Microbiol. 48, 541–545. https://doi.org/10.1007/s12275-010-0221-9 (2010).
Reis, V. M., Olivares, F. L. & Döbereiner, J. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J. Microbiol. Biotechnol. 10, 401–405. https://doi.org/10.1007/BF00144460 (1994).
Gauri, S. S., Mandal, S. M., Mondal, K. C., Dey, S. & Pati, B. R. Enhanced production and partial characterization of an extracellular polysaccharide from newly isolated Azotobacter sp. SSB81. Bioresour. Technol. 100, 4240–4243. https://doi.org/10.1016/j.biortech.2009.03.064 (2009).
Chun, J. et al. EzTaxon: A web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbi. 57, 2259–2261. https://doi.org/10.1099/ijs.0.64915-0 (2007).
da Silva, S. Q. Proposta para avaliação do controle biológico da cochonilha Diaspis echinocacti (Bouché, 1833) (Homoptera, Diaspididae) da palma forrageira em Pernambuco. Mater’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil (1991).
Finney, D. J. Probit Analysis (Cambridge University Press, Cambridge, 1971).
Ait Bahadou, S., Ouijja, A., Karfach, A., Tahiri, A. & Lahlali, R. New potential bacterial antagonists for the biocontrol of fire blight disease (Erwinia amylovora) in Morocco. Microb. Pathog. 117, 7–15. https://doi.org/10.1016/j.micpath.2018.02.011 (2018).
Amine, E., Sijilmassi, B., Maafa, I., Allal, D. & Ahmed, S. Biocontrol activity of Bacillus, Paenibacillus and Pseudomonas against Fusarium wilt of chickpea in Morocco. Acta Agric. Scand. Sect. B Soil Plant Sci. 72, 847–859. https://doi.org/10.1080/09064710.2022.2100819 (2022).
Gümüşsoy, A. et al. Identification and biocontrol potential of entomopathogenic nematodes and their endosymbiotic bacteria in apple orchards against the codling moth. Cydia pomonella (L.) (Lepidoptera: Tortricidae). Insects 13, 1085. https://doi.org/10.3390/insects13121085 (2022).
Cimen, H., Touray, M., Gulsen, S. H. & Hazir, S. Natural products from Photorhabdus and Xenorhabdus: Mechanisms and impacts. Appl. Microbiol. Biotechnol. 106, 4387–4399. https://doi.org/10.1007/s00253-022-12023-9 (2022).
Hasan, M. A., Ahmed, S., Mollah, M. M. I., Lee, D. & Kim, Y. Variation in pathogenicity of different strains of Xenorhabdus nematophila; Differential immunosuppressive activities and secondary metabolite production. J. Invertebr. Pathol. 166, 107221. https://doi.org/10.1016/j.jip.2019.107221 (2019).
Sharma, A., Thakur, D. R., Kanwar, S. & Chandla, V. K. Diversity of entomopathogenic bacteria associated with the white grub, Brahmina coriacea. J. Pest. Sci. 86, 261–273. https://doi.org/10.1007/s10340-012-0459-5 (2013).
Lacey, L. A. Entomopathogens used as microbial control agents. In Microbial Control of Insect and Mite Pests: From Theory to Practice 3–12 (Academic Press, 2017).
Subkrasae, C. et al. Larvicidal activity of Photorhabdus and Xenorhabdus bacteria isolated from insect parasitic nematodes against Aedes aegypti and Aedes albopictus. Acta Trop. 235, 106668. https://doi.org/10.1016/j.actatropica.2022.106668 (2022).
Battistuzzi, F. U. & Hedges, S. B. A major clade of prokaryotes with ancient adaptations to life on land. Mol. Biol. Evol. 26, 335–343. https://doi.org/10.1093/molbev/msn247 (2009).
Parte, A. C. LPSN—List of Prokaryotic names with Standing in Nomenclature (bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 68, 1825–1829. https://doi.org/10.1099/ijsem.0.002786 (2018).
Weller, D. M. et al. Induced systemic resistance in Arabidopsis thaliana against Pseudomonas syringae pv. tomato by 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Phytopathology 102, 403–412. https://doi.org/10.1094/PHYTO-08-11-0222 (2012).
Hirano, S. S. & Upper, C. D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae-a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64, 624–653. https://doi.org/10.1128/MMBR.64.3.624-653.2000 (2000).
Loper, J. E. et al. Comparative genomics of plant-associated Pseudomonas spp.: Insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 8, e1002784. https://doi.org/10.1371/journal.pgen.1002784 (2012).
Vodovar, N. et al. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. U. S. A. 102, 11414–11419. https://doi.org/10.1073/pnas.0502240102 (2005).
Höfte, M. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant disease. In Microbial Bioprotectants for Plant Disease Management (Burleigh Dodds, 2021).
Peix, A., Ramírez-Bahena, M.-H. & Velázquez, E. Historical evolution and current status of the taxonomy of genus Pseudomonas. Infect. Genet. Evol. 9, 1132–1147. https://doi.org/10.1016/j.meegid.2009.08.001 (2009).
Erickson, D. L., Lines, J. L., Pesci, E. C., Venturi, V. & Storey, D. G. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72, 5638–5645. https://doi.org/10.1128/IAI.72.10.5638-5645.2004 (2004).
Mahar, A. N. et al. Use of entomopathogenic bacterium Pseudomonas putida (Enterobacteriaceae) and its secretion against greater wax moth, Galleria mellonella Pupae. J. Entomol. 2, 77–85. https://doi.org/10.3923/je.2005.77.85 (2005).
Karthiba, L. et al. PGPR and entomopathogenic fungus bioformulation for the synchronous management of leaffolder pest and sheath blight disease of rice. Pest Manag. Sci. 66, 555–564. https://doi.org/10.1002/ps.1907 (2010).
Kim, S. K. et al. Insecticidal activity of rhamnolipid isolated from pseudomonas sp. EP-3 against green peach aphid (Myzus persicae). J. Agric. Food Chem. 59, 934–938. https://doi.org/10.1021/jf104027x (2011).
Ruffner, B. et al. Oral insecticidal activity of plant-associated pseudomonads: Insecticidal activity of pseudomonas. Environ. Microbiol. 15, 751–763. https://doi.org/10.1111/j.1462-2920.2012.02884.x (2013).
Chen, W.-J. et al. Characterization of an insecticidal toxin and pathogenicity of Pseudomonas taiwanensis against insects. PLOS Pathogens 10, e1004288. https://doi.org/10.1371/journal.ppat.1004288 (2014).
Smee, M. R., Baltrus, D. A. & Hendry, T. A. Entomopathogenicity to two hemipteran insects is common but variable across epiphytic Pseudomonas syringae strains. Front. Plant Sci. 8, 2149 (2017).
Wei, J.-Z. et al. A selective insecticidal protein from Pseudomonas mosselii for corn rootworm control. Plant Biotechnol. J. 16, 649–659. https://doi.org/10.1111/pbi.12806 (2018).
Vacheron, J. et al. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. ISME J. 13, 1318–1329. https://doi.org/10.1038/s41396-019-0353-8 (2019).
Panayidou, S. et al. Pseudomonas aeruginosa core metabolism exerts a widespread growth-independent control on virulence. Sci. Rep. 10, 9505. https://doi.org/10.1038/s41598-020-66194-4 (2020).
Kwon, S. W. et al. Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. and Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int. J. Syst. Evol. Microbiol. 53, 21–27. https://doi.org/10.1099/ijs.0.02326-0 (2003).
Hultberg, M., Alsberg, T., Khalil, S. & Alsanius, B. Suppression of disease in tomato infected by Pythium ultimum with a biosurfactant produced by Pseudomonas koreensis. BioControl 55, 435–444. https://doi.org/10.1007/s10526-009-9261-6 (2010).
Hultberg, M., Bengtsson, T. & Liljeroth, E. Late blight on potato is suppressed by the biosurfactant-producing strain Pseudomonas koreensis 2.74 and its biosurfactant. BioControl 55, 543–550. https://doi.org/10.1007/s10526-010-9289-7 (2010).
Rangel, L. I. et al. Characterization of toxin complex gene clusters and insect toxicity of bacteria representing four subgroups of Pseudomonas fluorescens. PLOS ONE 11, e0161120. https://doi.org/10.1371/journal.pone.0161120 (2016).
Olcott, M. H. et al. Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS ONE 5, e12504. https://doi.org/10.1371/journal.pone.0012504 (2010).
Lin, H. et al. Genome sequence of Pseudomonas koreensis CRS05-R5, an antagonistic bacterium isolated from rice paddy field. Front. Microbiol. 7, 1756 (2016).
Ichikawa, N. et al. DoBISCUIT: A database of secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 41, D408–D414. https://doi.org/10.1093/nar/gks1177 (2013).
Acknowledgements
This research was supported by the Mohamed VI Polytechnic University and the Moroccan foundation for advanced science innovation and research.
Author information
Authors and Affiliations
Contributions
K.El., C.R., I.M.K., M.El., R.B., A.A. and B.S. conceived and designed research. K.El. and C.R. and A.A. conducted experiments. K.El. contributed new reagents or analytical tools. K.El. and I.M.K. analyzed data. K.El. and C.R. wrote the manuscript. K.El. and C.R. contributed equally in the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Fakhouri, K., Ramdani, C., Aasfar, A. et al. Isolation, identification and pathogenicity of local entomopathogenic bacteria as biological control agents against the wild cochineal Dactylopius opuntiae (Cockerell) on cactus pear in Morocco. Sci Rep 13, 21647 (2023). https://doi.org/10.1038/s41598-023-48976-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-48976-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.