ABSTRACT
Sugarcane orange rust caused by Puccinia kuehnii has recently become an important disease in sugarcane crops and its spread is causing great concern to growers. In this study, we analyzed spores from symptomatic orange rust sugarcane leaves collected in multiple locations in Cuba in a 4-year-period in order to characterize morphological traits of P. kuehnii, establish an adequate molecular technique to characterize it, and determine its infection court in sugarcane. Orange rust caused by P. kuehnii was confirmed by polymerase chain reaction (PCR) and morphological characterization. AFLP markers detected high diversity in P. kuenhnii samples. Sequencing of rDNA regions, as expected, did not reveal differences and SSR markers designed for P. melanocephala could not be transferred to P. kuehnii. In addition to stomata, entry through prickles was also detected as a new infection court in sugarcane. Although the presence of pustules on the adaxial leaf surface was frequently detected, no clear correlation between this presence and density of stomata and/or prickles was found.
AFLP; rDNA; SSR; infection courts
Introduction
Sugarcane is the main sugar crop in the world, providing approximately 70 % of total sugar production, and is gaining increasing importance as a bioenergy supply. Among the fungal diseases affecting this crop are rusts caused by Puccinia melanocephala H. and P. Sydow (brown rust), and P . kuehnii (Krüger) Butler (orange rust), both occur worldwide. Orange rust was of relatively little economic importance until about a decade ago, when it caused a severe outbreak in Australia ( Braithwaite et al., 2009Braithwaite, K.S.; Croft, B.J.; Magarey, R.C.; Scharaschkin, T. 2009. Phylogenetic placement of the sugarcane orange rust pathogen Puccinia kuehnii in a historical and regional context. Australasian Plant Pathology 38: 380-388. ); probably why few studies have been conducted to characterize the disease in detail. In the western hemisphere, P . kuehnii was first detected in Florida, the United States, in 2007 ( Comstock et al., 2008Comstock, J.C.; Sood, S.G.; Glynn, N.C.; Shine Jr., J.M.; McKemy, J.M.; Castlebury, L.A. 2008. First report of Puccinia kuehnii , causal agent of orange rust of sugarcane in the United States and western hemisphere. Plant Disease 92: 175. ), but it spread afterwards throughout Central and South America. There is a third sugarcane rust, known as tawny rust ( Macruropyxis fulva sp. nov.), which causes similar symptoms to orange rust, but it is currently confined to South Africa, Mozambique and Zimbabwe ( Martin et al., 2015Martin, L.A.; Evans, D.L.; Rutherford, R.S.; McFarlane, S.A. 2015. Tawny rust: an update on the new species of rust infecting sugarcane in Southern Africa. Proceedings of the South African Sugar Technologists Association 88: 309-313. ).
The most effective way to control sugarcane rusts is the use of resistant crop varieties; however, some fungicides are a temporary control of the disease. A better knowledge on resistance requires a thorough understanding of pathogen genetic diversity as well as the mode of interaction with different sugarcane cultivars. It is well known that resistance breakdown is probably due to the occurrence of new pathogen genotypes ( Pillay et al., 2005Pillay, L.; McFarlane, S.A.; Rutherford, R.S. 2005. A preliminary report on genetic diversity in populations of sugarcane rust in KwaZulu-Natal. Proceedings of the South African Sugar Technologists Association 79: 132-136. ).
Orange rust is a relatively new disease in Cuba ( Pérez-Vicente et al., 2010Pérez-Vicente, L.; Martín-Triana, E.L.; Barroso, F.; Martínez-de la Parte, E.; Borrás-Hidalgo, O.; Hernández Estévez, I. 2010. Definitive identification of orange rust of sugarcane caused by Puccinia kuehnii in Cuba. Plant Patholology 59: 804. ), where environmental conditions, such as frequent alternation between rainy and dry days with prolonged dew periods, favor infection, spore production and disease spread. In addition, the diverse varietal composition of sugarcane planted on the island could favor pathogen variability, although no previous study has evaluated pathogen diversity in Cuba.
Therefore, this study aimed to: i) morphologically characterize P . kuehnii ; ii) determine a molecular technique to estimate genetic diversity of this pathogen in Cuba; and iii) define infection courts for P . kuehnii on sugarcane.
Materials and Methods
Sample collection
Orange rust samples, obtained from the third leaf from the top of multiple sugarcane varieties, were collected from 2011 to 2014 at five different locations in Cuba ( Table 1 ).
Molecular identification of the causal agent of rust symptoms
DNA was extracted from all samples using the protocol proposed by Aljanabi et al. (1999)Aljanabi, S.M.; Forget, L.; Dookun, A. 1999. An improved and rapid protocol for the isolation of polysaccharide and polyphenol: free sugarcane DNA. Plant Molecular Biology Reporter 17: 281. with minor modifications. The identity of the causal agent was verified by PCR using specific primers for P. kuehnii (PkPmF/Pk1R; Glynn et al., 2010Glynn, N.C.; Dixon, L.J.; Castlebury, L.A.; Szabo, L.J.; Comstock, J.C. 2010. PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P . melanocephala and detection of a SNP associated with geographical distribution in P . kuehnii . Plant Patholology 59: 703-711. ), P . melanocephala (Pm1F/Pm1R; Glynn et al., 2010Glynn, N.C.; Dixon, L.J.; Castlebury, L.A.; Szabo, L.J.; Comstock, J.C. 2010. PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P . melanocephala and detection of a SNP associated with geographical distribution in P . kuehnii . Plant Patholology 59: 703-711. ) and Macruropyxis fulva sp. nov. (unpublished sequence, generously provided by S. McFarlane, SASRI, South Africa), since symptoms caused by P . kuehnii are often confused with those caused by P . melanocephala or M . fulva .
Morphological characterization of P . kuehnii
Free-hand cross-sections of leaves, fixed in formalin acetic acid-alcohol (FAA), were used for the morphological analysis of pustules ( D’Ambrogio de Argüeso, 1986D’Ambrogio de Argüeso, A. 1986. Manual of Techniques in Vegetal Histology = Manual de técnicas en histología vegetal. Southern Hemisphere, Buenos Aires, Argentina (in Spanish). ). Spores were harvested from pustules using a micropipette. Infected leaves and spores were observed and photographed under a light microscope (Leica DM 500). Selected plant samples were observed and photographed in a JEOL JSM35 CF scanning electron microscope. The morphological characteristics analyzed were: epidermal position of pustules, presence or absence of paraphyses, color, size, wall thickening and number of germ pores of spores.
Molecular characterization of P . kuehnii
Symptomatic leaves were cut into sections, immediately dried at room temperature to perform AFLP, SSR and sequence the rDNA of P . kuehnii . Spores were harvested by scraping about 60 leaves (two per plant) of each sugarcane variety. The DNA analysis was performed in 14 samples ( Table 1 ) with enough spores collected. A careful microscopic inspection was carried out to visualize purity of P . kuehnii spores in each sample, discarding any extraneous microorganism. Total genomic DNA was extracted from 10 mg of spores mixed with 10 mg of sterile sand by using a cetyltrimethyl ammonium bromide (CTAB) protocol adapted from Aljanabi et al. (1999)Aljanabi, S.M.; Forget, L.; Dookun, A. 1999. An improved and rapid protocol for the isolation of polysaccharide and polyphenol: free sugarcane DNA. Plant Molecular Biology Reporter 17: 281. with an additional cleaning step with phenol ( Racedo et al., 2015Racedo, J.; Perera. M.F.; Bertani, R.; Funes, C.; Gonzalez, V.; Cuenya, M.I.; D’Hont, A.; Welin, B.; Castagnaro, A.P. 2015. Molecular diagnostic of both brown and orange sugarcane rust and evaluation of sugarcane brown rust resistance in Tucumán, Argentina using molecular markers associated to Bru1 a broad-range resistance allele. Sugar Tech 18: 414-419. ). Two separate DNA extractions were performed for each sample.
AFLP assays were performed according to Vos et al. (1995)Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23: 4407-4414. with some minor modifications by using 16 primer pairs. Cy5.5-dCTP was included in the final amplification reactions in order to detect amplified DNA fragments using a LI-COR 4300 DNA Analyzer. Images were captured with slow scan laser at 700 nm and analyzed with the SAGATM software. DNA from P . melanocephala collected from the susceptible variety B4362 was included as an outgroup control sample. Each amplified fragment was scored considering presence and absence of a fragment in each sample, and transformed into either a 0 or 1 matrix. The similarity matrix was calculated by the Jaccard coefficient ( Sneath and Sokal, 1973Sneath, P.H.A.; Sokal, R.R. 1973. Numerical Taxonomy: The Principles and Practice of Numerical Classification. Freeman, San Francisco, CA, USA. ). The cluster analyses were carried out using the McQuitty similarity analysis (Unweighted Pair Group Method with Arithmetic Mean, UPGMA) ( Sneath and Sokal, 1973Sneath, P.H.A.; Sokal, R.R. 1973. Numerical Taxonomy: The Principles and Practice of Numerical Classification. Freeman, San Francisco, CA, USA. ). All traits were included in the analysis and calculations were carried out using the software InfoStat (v. 2009).
Seven SSR primer pairs developed by Peixoto et al. (2013)Peixoto Jr., R.F.; Figueira, A.V.O.; Landell, M.G.A.; Nunes, D.S.; Pinto, L.R.; Sanguino, A. 2013. Development and characterization of microsatellite markers for Puccinia melanocephala , causal agent of sugarcane brown rust. Proceedings International Society of Sugar Cane Technologists 28: 1-4. for P . melanocephala characterization were optimized for detection in the LI-COR 4300 DNA Analyzer and tested on the orange rust samples. Primer sequences (unpublished) and PCR conditions were provided by Peixoto et al. (2013)Peixoto Jr., R.F.; Figueira, A.V.O.; Landell, M.G.A.; Nunes, D.S.; Pinto, L.R.; Sanguino, A. 2013. Development and characterization of microsatellite markers for Puccinia melanocephala , causal agent of sugarcane brown rust. Proceedings International Society of Sugar Cane Technologists 28: 1-4. . DNA from P . melanocephala sample was included as an amplification control.
Three samples (C04-79.MY.13, C05-353.MY.13 and C89-147.MY.14), belonging to different branches in the dendrogram obtained with AFLP markers, were amplified with three pairs of primers: the P . kuehnii specific pair PkPmF/Pk1R and the two fungal generic primers, NL1/NL4 ( O’Donnell, 1993O’Donnell, K. 1993. Fusarium and its near relatives. p. 225-233. In: Reynolds, D.R.; Taylor, J.W., eds. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK. ) and LR12RE/5SRNA ( James et al., 2001James, T.Y.; Moncalvo, J.; Li, S.; Vilgalys, R. 2001. Polymorphism at the ribosomal DNA spacers and its relation to breeding structure of the widespread mushroom Schizophyllum commune. Genetics 157: 149-161. ). Amplification products were gel purified using the QIAquick Gel Extraction Kit and cloned into the pGEM-T Easy vector. Nine clones were sequenced on an automated DNA Sequencer. Nucleotide sequences obtained in both directions were used to create the complete sequence by using DNAMan software. The regions were aligned and their phylogeny determined by ClustalX (v. 2.0.) using the neighbor-joining option with a bootstrap analysis of 1,000 random replications. At least one DNA sequence of P . kuehnii deposited in GenBank from each country and one of P . melanocephala were included in the analysis.
Characterization of P . kuehnii mode of entry
Urediniospores of P . kuehnii were harvested by scraping naturally infected leaves of the susceptible sugarcane cultivar C04-79 one day before inoculation. Viability was determined by plating on 1 % water agar overnight at room temperature. A suspension of approximately 1 × 104 viable urediniospores ml1 ( Sood et al., 2009Sood, S.G.; Comstock, J.C.; Glynn, N.C. 2009. Leaf whorl inoculation method for screening sugarcane rust resistance. Plant Disease 93: 1335-1340. ) was used to inoculate 20 healthy plants of cultivar C04-79 produced from single-bud cuttings in the greenhouse. Inoculum was applied on 40 to 60-day-old plants with four fully expanded leaves by manually spraying both sides of leaves. After inoculation, all sprayed plants were placed in a humid chamber at 25 °C in the dark to keep leaf wetness. Twenty-four hours later, plants were transferred to chambers with a 12 h d1 photoperiod for 14 d at room temperature. The penetration of P . kuehnii was observed under a light microscope in +1 leaf from each of five plants at different times after inoculation. As the presence of pustules was also detected on the adaxial epidermis of the inoculated variety, the adaxial epidermis of 10 sugarcane varieties was examined microscopically on field-collected samples. Densities of stomata and prickles were measured and presence or absence of orange rust pustules was determined in ten samples per variety.
Results
Molecular identification of the causal agent of rust symptoms
From all symptomatic leaf samples, only the specific DNA fragment for P . kuehnii was amplified. No band was observed when using the primer pairs, specific for P . melanocephala and M . fulva in any of the samples, not even when the DNA amount was increased over tenfold.
Morphological characterization of P . kuehnii
All collected leaves showed small, elongated yellowish lesions towards the leaf base in the first stage of disease development. As the disease progressed, lesions turned brownish orange and pustules could be noticed. Pustules were longitudinally elongated, opened by the lateral border and pyriform ( Figure 1A ). They were mainly located on the abaxial epidermis although they were also frequently found on the adaxial surface of some of the sugarcane genotypes analyzed ( Figures 2A and 2B ). Paraphyses were usually inconspicuous and hyaline to pale brown ( Figures 1B and 2C ).
– Scanning electron microscope images of pustules and spores of Puccinia kuehnii. A) Longitudinally elongated pustules. B) Urediniospores and paraphyses. C-D) Different shapes of urediniospores. E) Arragement of echinulations on the surface of urediniospores. p = paraphyses. Scale bar: A = 100 µm, B = 20 µm, C = 11 µm, D-E = 3.5 µm.
– A) Open pustules of Puccinia kuehnii on abaxial and adaxial epidermis. B) Pustule details. C) Paraphyses. D-G) Differences in wall thickness of orange rust uredionispores. H-J) Pale green color and hyaline appearance spores. K) Brownish orange spore with green wall. ad.e. = adaxial epidermis. ab.e. = abaxial epidermis. Scale bar: A-B = 65 µm, C = 6 µm, D-K = 8 µm.
Opened rust pustule showed an abundance of urediniospores that varied in shape, color and size, representing a mixture of immature, developing and mature spores ( Figures 3A-C ). Urediniospores showed characteristic features of P . kuehnii: mostly obovoid or pyriform, sometimes ellipsoidal and even rectangular. Their walls usually showed a pronounced apical thickening, but some spores displayed a uniform wall thickness and were moderately echinulate with three to five germ pores ( Figures 1C-E, 2D-K ). Spores were orange, but they can also be pale green with a hyaline appearance ( Figures 2H-J and Figure 3A-C ). Spore size was highly variable, ranging from 34-47 µm × 25-34 µm for the typical orange spores to 33-43 µm × 22-30 µm for the green hyaline spores.
– Pustules of Puccinia kuehnii at different development stages. Typical orange spores, along with pale green color and hyaline appearance spores, can be observed. g.h.s. = green hyaline spores. o.s.: = orange spores. Scale bar: A = 50 µm, B = 75 µm, C = 28 µm.
Molecular characterization of P . kuehnii
Unique and reproducible amplification profiles of AFLP markers allowed characterization of rust samples. We identified 667 fragments with 16 pairs of primers used, which detected 89 % of polymorphism. P . melanocephala could be clearly differentiated from P . kuehnii samples since the former grouped separately from the rest of the samples in the dendrogram ( Figure 4 ). Genetic similarities for P . kuehnii samples ranged between 0.27 and 0.64 using the Jaccard coefficient. P . kuehnii samples grouped into three main clusters (divided at 0.27 and 0.31 of similarity). A weak correlation of AFLP results was observed between samples with respect to their geographical origin and the sampling date.
– Phenogram of fourteen Puccinia kuehnii characterized samples and one Puccinia melanocephala outgroup control (B4362) based on the analysis of 667 markers produced from 16 AFLP primer pairs when using Jaccard coefficient and UPGMA clustering method with InfoStat program, presented as distance (1-S; S = similarity). Names indicate the sugarcane genotype and the location (HL = Holguín, MY = Mayabeque and VC = Villa Clara) and year (12: 2012; 13: 2013 and 14: 2014) of sampling. Lines indicate 0.64, 0.31 and 0.27 of similarity, respectively.
No DNA amplification of orange rust collected samples was obtained with the seven SSR primer pairs. Even when restrictiveness of PCR reactions was lowered by doubling the concentration of Taq DNA polymerase and step-wise decreasing the annealing temperature ten degrees (0.5 °C in each step), no amplification was obtained. In contrast, the P . melanocephala control DNA was amplified with all seven-primer pairs. When restrictiveness was lowered, the number of bands increased for the P . melanocephala sample.
Three samples were amplified with three pairs of primers: the P . kuehnii specific pair PkPmF/Pk1R (ITS1-5.8S -ITS2 region) and the two fungus generic primers, NL1/NL4 (LSU region) and LR12RE/5SRNA (LSU-IGS1-5S-IGS2 region). The DNA sequences obtained for each of the three genomic regions of each sample (C04-79.MY.13: KP203314 (ITS), KP203317 (LSU), KP203320 (IGS); C05-353.MY.13: KP203315 (ITS), KP203318 (LSU), KP203321 (IGS) and C89-147.MY.14: KP203316 (ITS), KP203319 (LSU), KP203322 (IGS)) did not show significant differences between them when they were aligned. The phylogenetic study conducted including sequences of other countries revealed the same behavior for each of the three characterized regions ( Figures 5A-C ). The three Cuban samples clustered together in the main group that includes most P . kuehnii world isolates. P . melanocephala grouped separately from the rest of the isolates.
– Phylogenetic tree of Puccinia kuehnii samples based on internal transcribed spacer (ITS, A), large subunit (LSU, B) and intergeneric spacer (IGS, C) nucleotide sequences constructed by the neighbor joining method with 1,000 bootstrap replications. Sequences from GenBank of Puccinia kuehnii and one of Puccinia melanocephala as outgroup control were included.
Characterization of the entry mode of P . kuehnii
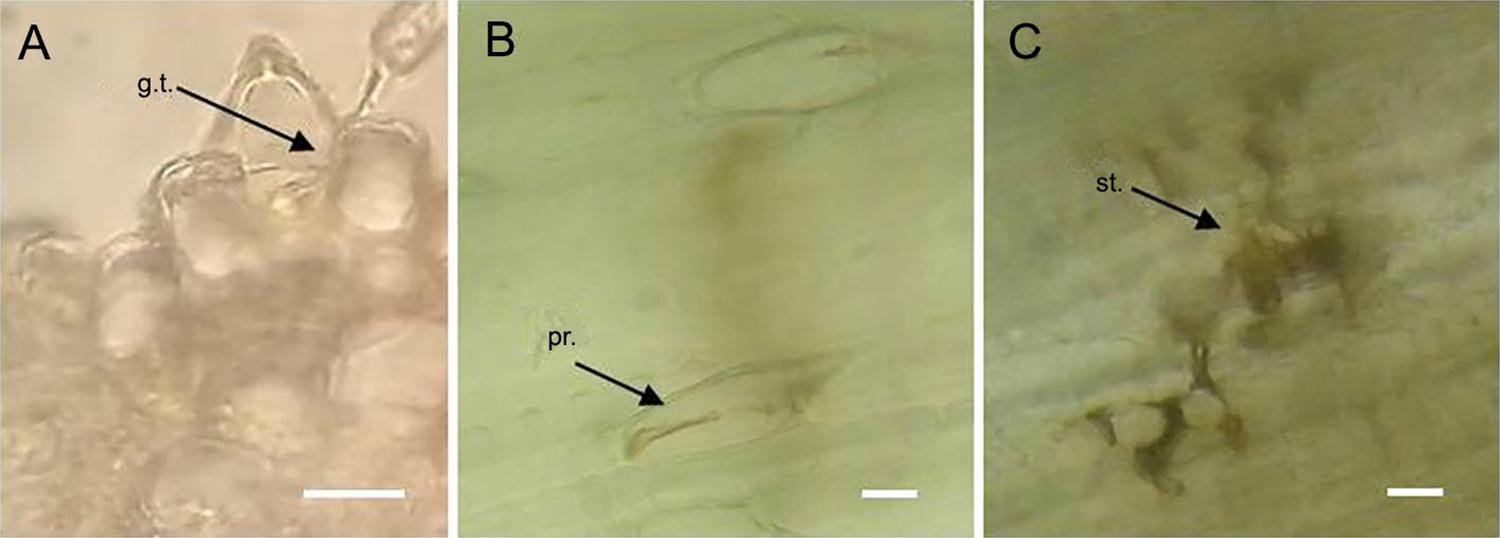
The common germ-tube entry site reported for P . kuehnii through stomata was detected; however, entry through prickles was observed ( Figure 6A ). Cell alterations at both wall and cytoplasmatic content observed in infected prickles were similar to those observed in infected stomata ( Figures 6B and 6C ).
– Puccinia kuehnii penetration points in sugarcane leaves. A) Germ tube entering through a prickle in a transversal cross section of the leaf one day-post inoculation. B) Alterations of an invaded prickle in paradermal view 29 day-post inoculation. C) Alterations of an invaded stoma in paradermal view 29 day-post inoculation. g.t. = germ tube; pr. = prickle; st. = stoma. Scale bar: 20 µm.
Densities of stomata and prickles on the adaxial leaf surface of sugarcane were measured ( Table 2 ); however, no clear correlation between the presence of the pustules and densities could be detected. Noticeably, the C04-79 variety that presented pustules on both surfaces when it was inoculated under controlled growth conditions was free of pustules on the adaxial side of the leaves in field-collected samples. This could be due to a high inoculum pressure in the experiment under controlled growth conditions that favored pathogen entry on both leaf sides, while such favorable conditions may be absent in the field. This variety presents a high density of stomata on the adaxial side, which supposedly should facilitate pathogen entry on this side. The C86-456 variety has the highest densities of stomata and prickles on the leaf adaxial side, and orange rust pustules were observed in the collected field samples on this leaf surface. Genotype C89-559 also had pustules on the adaxial surface, whereas the density of stomata and prickles was low. C2002-222, in contrast, had high densities of stomata and prickles on the adaxial side, but no pustules on this leaf side were detected.
– Average stoma and prickle densities and presence of orange rust pustules on the adaxial epidermis of ten sugarcane field collected samples.
Discussion
Symptoms of orange rust sugarcane in Cuba were caused by the fungal pathogen P . kuehnii . Its presence was confirmed by specific PCR amplification of DNA before the morphological and molecular characterization in order to ensure sample identity. Although tawny rust and orange rust cause very similar symptoms ( Martin et al., 2015Martin, L.A.; Evans, D.L.; Rutherford, R.S.; McFarlane, S.A. 2015. Tawny rust: an update on the new species of rust infecting sugarcane in Southern Africa. Proceedings of the South African Sugar Technologists Association 88: 309-313. ), the causal agent of the former was not detected in any Cuban sample tested.
Regarding morphological characterization the presence of pustules in both epidermises was frequently occurring, an observation that has not been previously described in the literature. The analysis of uredioniospores revealed an extensive variety of shapes, sizes they cover a wide range and colors as reported in previous studies (Virtudazo et al., 2001b). The presence of paraphyses is a character reported as a rare occurrence in Australia (Magarey, 2010), occasionally in studies on orange rust in North America ( Dixon et al., 2010Dixon, L.J.; Castlebury, L.A.; Aime, M.C.; Glynn, N.C.; Comstock, J.C. 2010. Phylogenetic relationships of sugarcane rust fungi. Mycological Progress 9: 459-468. ), but not detected in studies conducted in Colombia ( Cadavid et al., 2012Cadavid, M.; Ángel, J.C.; Victoria, J.I. 2012. First report of orange rust of sugarcane caused by Puccinia kuehnii in Colombia. Plant Disease 96: 143. ). The shape and color of paraphyses observed in the Cuban samples were in agreement with those previously observed.
Keiper et al. (2003)Keiper, F.J.; Hayden, M.J.; Park, R.F.; Welling, C.R. 2003. Molecular genetic variability of Australian isolates of five cereal rust pathogens. Mycological Research 107: 545-556. suggested that DNA fingerprinting techniques such as AFLP and SSR could be more suitable than other assessments for detecting intra-specific variation. In our study, the AFLP technique was highly useful when characterizing P . kuehnii samples. Although the number of samples included in the study was reduced, the method revealed a high genetic diversity of the pathogen. This high diversity in the Cuban samples could be explained by long-distance dispersion of rust spores in air currents (Magarey, 2010) and the wide varietal spectrum in Cuba, where more than 99 varieties are planted. According to the law, no sugarcane variety can exceed 20 % of the cultivated area of the country ( La O et al., 2018La O, M.; Perera, M.F.; Bertani, R.P.; Acevedo, R.; Arias, M.E.; Casas, M.A.; Pérez, J.; Puchades, Y.; Rodríguez, E.; Alfonso, I.; Castagnaro, A.P. 2018. An overview of sugarcane brown rust in Cuba. Scientia Agricola 75: 233-238. ), then, the risk that the pathogen could specialize and overcome resistant genes is somewhat mitigated. In that sense, the variability of spores might result from pathogen adaptation to the large number of sugarcane varieties planted.
Peixoto et al. (2013)Peixoto Jr., R.F.; Figueira, A.V.O.; Landell, M.G.A.; Nunes, D.S.; Pinto, L.R.; Sanguino, A. 2013. Development and characterization of microsatellite markers for Puccinia melanocephala , causal agent of sugarcane brown rust. Proceedings International Society of Sugar Cane Technologists 28: 1-4. suggested that SSR designed for P . melanocephala could be transferred to and used for P . kuehnii . However, no amplification was achieved by using these SSR primers in the Cuban samples. SSR specific primers need to be developed to characterize P . kuehnii samples, because it would be advantageous to extract DNA directly from sugarcane leaves.
The phylogenetic relationships of three different samples were generated from sequence data from three rDNA regions: two variable spacers (internal transcribed spacer, ITS and intergeneric spacer, IGS) and a more conserved coding region (large subunit, LSU) than the spacers. However, rDNA sequences of these three samples belonging to different clusters in the AFLP analysis did not show any polymorphism for each region sequenced. Similar results were previously obtained for P . kuehnii samples (Virtudazo et al., 2001a; Braithwaite et al., 2009Braithwaite, K.S.; Croft, B.J.; Magarey, R.C.; Scharaschkin, T. 2009. Phylogenetic placement of the sugarcane orange rust pathogen Puccinia kuehnii in a historical and regional context. Australasian Plant Pathology 38: 380-388. ). Considering that rDNA represents only a small fragment of the entire genome, it may not reveal pathogen genetic diversity as the other molecular technique employed in the present study.
Germ tubes of rust spores in general form an appressorium over the stomatal aperture, which is necessary for the infection of the host tissue ( Sotomayor et al., 1983Sotomayor, I.A.; Purdy, L.H.; Trese, A.T. 1983. Infection of sugarcane leaves by Puccinia melanocephala. Phytopathology 73: 695-699. ). An exception to this typical pattern, which involves cuticular penetration of the germ tubes of urediniospores between epidermal cells, has been reported especially in rusts of tropical dicotyledonous hosts (Hunt et al., 1968). In the present work, entry of P . kuehnii through stomata was confirmed, but entry through prickles was also found. This entry mode has not been reported previously and may be unique to certain sugarcane variety/ P . kuehnii combinations.
Uredinia develops mainly on the abaxial leaf surface, but certain cultivars may also have some uredinia on the adaxial surface ( Purdy, 2014Purdy, L.H. 2014. Sugarcane rusts. p. 237-259. In: Roelfs, A.P.; Bushnell, W.R., eds. The cereal rusts: diseases, distribution, epidemiology and control. Academic Press, Orlando, FL, USA. ), as observed in the present study for some sugarcane field collected samples. The presence of pustules on the adaxial epidermis could be correlated with leaf surface anatomical characteristics in each variety. However, in our study, we found no clear correlation between pustules on the adaxial surface and stoma or prickle densities. Some varieties with high density of both characters did not have pustules on the adaxial surface; other characteristics may have played a more important role, such as the thickness of the cell wall and cuticle, as reported by P . melanocephala resistance in sugarcane genotypes ( Acevedo et al., 2011Acevedo, R.; Luque, A.C.; Alfonso, I.; Debes, M.; Rodríguez, E.; La O, M. 2011. Leaf morphological characters in sugarcane cultivars with different resistant grades to brown rust. Acta Microscopica 20 Supplement B. ).
In conclusion, the present work used molecular techniques to characterize and estimate genetic diversity of P . kuehnii , revealing a highly variable pathogen population in Cuba and discovering a new infection court that may be of epidemiological significance.
Acknowledgements
This project was supported by Estación Experimental Agroindustrial Obispo Colombres (EEAOC), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Universidad Nacional de Tucumán (UNT), Instituto de Investigaciones de la Caña de Azúcar (INICA) and Fondo Argentino de Cooperación Sur-Sur y triangular (FOAR). M.F. Perera and A.P. Castagnaro are CONICET members and R.P. Bertani and M.A. Debes, CONICET fellows. We thank C.R. Gonçalves (Centro de Tecnologia Canavieira, Brazil) for the positive control for P . kuehnnii , S. McFarlane (South African Sugarcane Research Institute) for the positive control, protocol and primer sequences for the causal agent of tawny rust detection, R.F. Peixoto (Universidade de São Paulo, Brazil) for the SSR primer sequences and L. Montivero for reviewing the manuscript English version.
References
- Acevedo, R.; Luque, A.C.; Alfonso, I.; Debes, M.; Rodríguez, E.; La O, M. 2011. Leaf morphological characters in sugarcane cultivars with different resistant grades to brown rust. Acta Microscopica 20 Supplement B.
- Aljanabi, S.M.; Forget, L.; Dookun, A. 1999. An improved and rapid protocol for the isolation of polysaccharide and polyphenol: free sugarcane DNA. Plant Molecular Biology Reporter 17: 281.
- Braithwaite, K.S.; Croft, B.J.; Magarey, R.C.; Scharaschkin, T. 2009. Phylogenetic placement of the sugarcane orange rust pathogen Puccinia kuehnii in a historical and regional context. Australasian Plant Pathology 38: 380-388.
- Cadavid, M.; Ángel, J.C.; Victoria, J.I. 2012. First report of orange rust of sugarcane caused by Puccinia kuehnii in Colombia. Plant Disease 96: 143.
- Comstock, J.C.; Sood, S.G.; Glynn, N.C.; Shine Jr., J.M.; McKemy, J.M.; Castlebury, L.A. 2008. First report of Puccinia kuehnii , causal agent of orange rust of sugarcane in the United States and western hemisphere. Plant Disease 92: 175.
- D’Ambrogio de Argüeso, A. 1986. Manual of Techniques in Vegetal Histology = Manual de técnicas en histología vegetal. Southern Hemisphere, Buenos Aires, Argentina (in Spanish).
- Dixon, L.J.; Castlebury, L.A.; Aime, M.C.; Glynn, N.C.; Comstock, J.C. 2010. Phylogenetic relationships of sugarcane rust fungi. Mycological Progress 9: 459-468.
- Glynn, N.C.; Dixon, L.J.; Castlebury, L.A.; Szabo, L.J.; Comstock, J.C. 2010. PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P . melanocephala and detection of a SNP associated with geographical distribution in P . kuehnii . Plant Patholology 59: 703-711.
- Hunt, P. 1968. Cuticular penetration by germinating uredospores. The British Mycological Society 51: 103-107.
- James, T.Y.; Moncalvo, J.; Li, S.; Vilgalys, R. 2001. Polymorphism at the ribosomal DNA spacers and its relation to breeding structure of the widespread mushroom Schizophyllum commune. Genetics 157: 149-161.
- Keiper, F.J.; Hayden, M.J.; Park, R.F.; Welling, C.R. 2003. Molecular genetic variability of Australian isolates of five cereal rust pathogens. Mycological Research 107: 545-556.
- La O, M.; Perera, M.F.; Bertani, R.P.; Acevedo, R.; Arias, M.E.; Casas, M.A.; Pérez, J.; Puchades, Y.; Rodríguez, E.; Alfonso, I.; Castagnaro, A.P. 2018. An overview of sugarcane brown rust in Cuba. Scientia Agricola 75: 233-238.
- Magarey, R.C. 2010. Orange rust disease of sugarcane. Proceedings International Society of Sugar Cane Technologists 27: 1-9.
- Martin, L.A.; Evans, D.L.; Rutherford, R.S.; McFarlane, S.A. 2015. Tawny rust: an update on the new species of rust infecting sugarcane in Southern Africa. Proceedings of the South African Sugar Technologists Association 88: 309-313.
- O’Donnell, K. 1993. Fusarium and its near relatives. p. 225-233. In: Reynolds, D.R.; Taylor, J.W., eds. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, UK.
- Peixoto Jr., R.F.; Figueira, A.V.O.; Landell, M.G.A.; Nunes, D.S.; Pinto, L.R.; Sanguino, A. 2013. Development and characterization of microsatellite markers for Puccinia melanocephala , causal agent of sugarcane brown rust. Proceedings International Society of Sugar Cane Technologists 28: 1-4.
- Pérez-Vicente, L.; Martín-Triana, E.L.; Barroso, F.; Martínez-de la Parte, E.; Borrás-Hidalgo, O.; Hernández Estévez, I. 2010. Definitive identification of orange rust of sugarcane caused by Puccinia kuehnii in Cuba. Plant Patholology 59: 804.
- Pillay, L.; McFarlane, S.A.; Rutherford, R.S. 2005. A preliminary report on genetic diversity in populations of sugarcane rust in KwaZulu-Natal. Proceedings of the South African Sugar Technologists Association 79: 132-136.
- Purdy, L.H. 2014. Sugarcane rusts. p. 237-259. In: Roelfs, A.P.; Bushnell, W.R., eds. The cereal rusts: diseases, distribution, epidemiology and control. Academic Press, Orlando, FL, USA.
- Racedo, J.; Perera. M.F.; Bertani, R.; Funes, C.; Gonzalez, V.; Cuenya, M.I.; D’Hont, A.; Welin, B.; Castagnaro, A.P. 2015. Molecular diagnostic of both brown and orange sugarcane rust and evaluation of sugarcane brown rust resistance in Tucumán, Argentina using molecular markers associated to Bru1 a broad-range resistance allele. Sugar Tech 18: 414-419.
- Sneath, P.H.A.; Sokal, R.R. 1973. Numerical Taxonomy: The Principles and Practice of Numerical Classification. Freeman, San Francisco, CA, USA.
- Sood, S.G.; Comstock, J.C.; Glynn, N.C. 2009. Leaf whorl inoculation method for screening sugarcane rust resistance. Plant Disease 93: 1335-1340.
- Sotomayor, I.A.; Purdy, L.H.; Trese, A.T. 1983. Infection of sugarcane leaves by Puccinia melanocephala. Phytopathology 73: 695-699.
- Virtudazo, E.V.; Nakamura, H.: Kakishima, M. 2001a. Phylogenetic analysis of sugarcane rusts based on sequences of ITS, 5.8 S rDNA and D1/D2 regions of LSU rDNA. Journal of General Plant Pathology 67: 28-36.
- Virtudazo. E.V.; Nojima, H.; Kakishima, M. 2001b. Taxonomy of Puccinia species causing rust diseases on sugarcane. Mycoscience 42: 167-175.
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; van de Lee, T.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research 23: 4407-4414.
Edited by
Publication Dates
-
Publication in this collection
02 Sept 2019 -
Date of issue
2020
History
-
Received
14 Feb 2018 -
Accepted
04 Sept 2018